Cancer-related inflammation as predicting tool for treatment outcome in locally advanced and metastatic non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death and the non-small cell lung cancer (NSCLC) represents 80% of all cases (1). When diagnosed, in most cases the cancer is in locally advanced or metastatic stage of the disease (stage IIIB and IV). Although targeted therapeutics for lung cancer harboring activating mutations emerged during past years, for a large proportion of patients, platinum-based doublet chemotherapy is still the established first line therapeutic option (2).
Since the chemotherapy carries the burden of wide spectrum of considerable side effects the treatment decision should balance the benefits and undesirable effects of the treatment. One of the essential factors in deciding on treatment strategy is the expected prognosis.
In standard clinical practice, different clinical or pathological factors are used as prognostic tools helping to predict the outcome of the treatment such as pathohistological subtypes of the cancer, presence of different mutations, performance status (PS), weight loss, age or comorbidities (3-5). The most recognized prognostic factor, which is advised in guidelines for lung cancer treatment is PS (6). Still, the adequate predictive factor to sift the patients who will benefit the most from the chemotherapy, due to the heterogeneity of the disease, is yet to be found.
Systemic inflammation correlated with the carcinogenesis, tumor proliferation and dissemination, brings significant contribution in prognostic assessment of solid tumors. It has been shown that systemic symptoms related to the presence of cancer such as weight loss, anorexia, cachexia and anemia are inflammatory driven (7). The mechanism of the systemic inflammation in cancer is a result of systemic cytokine excess either triggered by the tumor itself or as part of the host’s innate response against cancer (8). Activation of the coagulation system is observed in patients with various malignancies and in lung cancer as well, and is also related to cancer derived humoral factors such as interleukin-6 (IL-6), interleukin-1 (IL-1), and macrophage colony-stimulating factor (9-13).
Aim of this study was to explore predictive value of the pre-treatment C-reactive protein (CRP), fibrinogen levels and their interaction for the response to frontline chemotherapy.
Methods
Trial design
This retrospective cohort study was done at University Hospital Centre Zagreb (UHC Zagreb), University Department for Lung Disease Jordanovac, Zagreb, Croatia. The study protocol was approved by UHC Zagreb Ethics committee and University of Zagreb Medical School Faculty Board, reference number 380-59-10106-13-51/13. All participants gave their written informed consent for participating in the study. The study was designed and executed in accordance with World Medical Association Declaration of Helsinki 2013 (14).
Participants
Patients of both gender, older than 40 years, diagnosed with locally advanced and metastatic NSCLC in stage IIIB and IV were eligible for the study. Exclusion criterion was brain metastasis at diagnosis. We chose a systematic, consecutive sample of patients by the order of their admission to the hospital. Patients with proven coexisting bacterial infection taking antibiotics at the time of diagnosis were excluded from the analysis.
Outcome
Disease control versus progression of the disease at re-evaluation after frontline chemotherapy. Response to chemotherapy was measured by comparison of the radiographic images of the chest before the treatment and at the completion of the treatment. Radiographic imaging used in this study comprised chest radiography in the anterior-posterior and lateral view and multi-slice computer tomography (MSCT) of thorax and upper abdomen. Chest radiography was used in regular check-ups after two cycles, as per standard clinical practice, while MSCT using RECIST criteria was used for the response assessment after completed first line therapy. Patients were divided in two groups regarding the radiological response to the treatment: in one were the patients who achieved disease control and in the other ones with disease progression. Disease control comprised complete response, partial response or stable disease achieved after first line chemotherapy confirmed by radiological assessment at re-evaluation. The laboratory test results registered and analyzed comprised: hemoglobin, leukocytes, fibrinogen, and CRP level pre-treatment and at the reevaluation. All patients had Eastern Cooperative Oncology Group (ECOG) PS 0 and 1.
Statistical methods
The level of statistical significance was set to P<0.05 and all confidence intervals (CIs) were given at 95% level. In all instances a two-tailed tests were used. The distributions were described by medians and interquartile ranges or counts and percentages. To access independent association of CRP, fibrinogen and their interaction with disease control at re-evaluation we did the multivariate (adjusted) binary logistic regression analysis. Odds ratios (OR) with their 95% CIs were given as measures of standardized effect sizes for disease control at re-evaluation. The moderating effect of fibrinogen on the association of CRP and chemotherapy outcome was analyzed by “Process”, release 2.12, Andrew F. Hayes, The Ohio State University, 2014. Fibrinogen value defining the region of statistically significant association of CRP and chemotherapy outcome was assessed by Johnson-Neyman technique as implemented in the “Process” (15). The probabilities of disease control at re-evaluation were calculated from odds as: probability = odds ratio/(1 + odds ratio). Statistical data analysis was done by R, version 3.0.1 (R Development Core Team).
Results
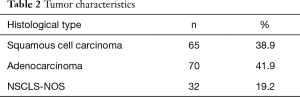
Total of 170 patients were eligible for the study, 127 (74.7%) were male and 43(25.3%) were female. Ages ranged between 40 and 82 years, with a median (interquartile range) age of 64 [57–70] years. Female patients were older than male patients to a moderate extent with median [interquartile range (IQR)] of 69 [57–73] years compared to 63 [57–69] years. Total of 70 (41.9%) of patients had adenocarcinoma, 65 (38.9%) had squamous cell carcinoma and 32 (19.2%) had NSCLC, not otherwise specified (NOS).
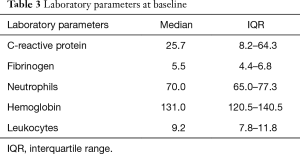
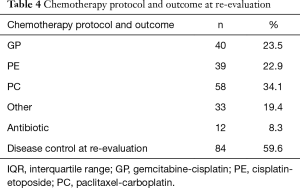
Data on histological type was missing for 3 (1.8%), on CRP for 12 (7.1%), fibrinogen for 68 (40%), neutrophils for 15 (8.8%), hemoglobin for 9 (5.3%), leukocytes for 9 (5.3%), antibiotic for 25 (14.7%) patients (Tables 1-4).

Full table
Full table
Full table
Full table
CRP and fibrinogen values were statistically significantly correlated at the beginning of chemotherapy (n=100; Spearmen’s rank correlation, ρ=0.67; P<0.001).
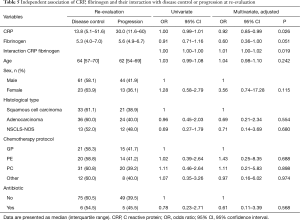
At re-evaluation after frontline chemotherapy disease control was achieved in 84/141 (59.6%) patients. After the adjustment for age, gender, cytological/histological type, chemotherapy protocol and antibiotic; CRP and the interaction of CRP and fibrinogen were independently significantly associated with disease control at re-evaluation.
There was a statistically significant difference in the median CRP level between the patients with disease control or progression at re-evaluation 13.8 vs. 30.0 mg/L respectively, P=0.026 (Table 5). In patients with disease control at re-evaluation, median (IQR) CRP changed from 13.8 (5.1–51.6) at baseline to 5.9 (1.7–15.6) at re-evaluation. In patients with disease progression at re-evaluation, median (IQR) CRP changed from 30.0 (11.6–60.7) at baseline to 29.1 (6.7–66.6) at re-evaluation. Change of CRP from base-line to re-evaluation was statistically significantly different in patients with disease control and progression of disease at reevaluation (P=0.003). In patients with disease control at re-evaluation, median (IQR) fibrinogen changed from 5.3 (4.0–7.0) at baseline to 4.1 (3.5–6.0) at re-evaluation. In patients with disease progression at re-evaluation, median (IQR) fibrinogen level changed from 5.6 (4.9–6.7) at baseline to 6.0 (4.4–7.2) at re-evaluation. Change of fibrinogen level from base-line to re-evaluation was statistically significantly different in patients with disease control or progression of disease at reevaluation (P=0.021).
Full table
Levels of neutrophils and hemoglobin were not significantly associated with disease control at re-evaluation (P=0.673, P=0.833 respectively) while levels of leukocytes were significantly associated with disease control (OR =0.92; 95% CI, 0.84–1.00; P=0.046).
Planned post-hoc analysis revealed that below the 10th percentile of fibrinogen values (≤3.3 g/L), higher CRP values were statistically significantly associated with progression of disease (Figure 1). At higher levels of fibrinogen, CRP level was not statistically significantly associated with disease control.
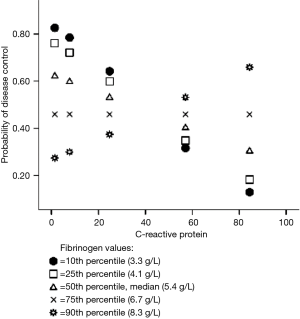
By Johnson-Neyman technique we found that bellow fibrinogen value of 3.5 g/L, CRP level is significantly associated with disease control and progression of the disease. Above this fibrinogen value the association of CRP and disease control is lost.
Discussion
In this study we explored the predictive value of CRP in interaction with fibrinogen on the chemotherapy response and found a relationship between levels of inflammatory and coagulation markers and the response to the given first line chemotherapy.
Locally advanced and metastatic NSCLC is one of the most aggressive types of malignant disease and the leading cause of cancer deaths for both men and women.
Emerging targeted therapy for cancers harboring different mutations is appropriate for small proportion of the patients and for majority of the patients chemotherapy is still the primary therapeutic strategy with the main goals of palliation and prolonging life. There is an ongoing quest for firm prognostic and predictive factors that can help select the patients who will benefit the most from the chosen chemotherapy.
A solid evidence of intersections between inflammation and cancer pathogenesis exists, demonstrating important tumor-promoting effects that immune cells have on neoplastic progression (16-19). Inflammation can contribute to cancer progression by producing different bioactive molecules to the tumor microenvironment that can stimulate growth, survival of cancer cells, angiogenesis, invasion and dissemination; it is considered as an emerging hallmark of cancer progression (16-18,20,21). Cancer related inflammation has specific cytokine signature of simultaneous immunostimulation and immunosuppression with increased concentrations of the cytokines macrophage migration inhibitory factor (MIF), tumor necrosis factor alpha (TNFα), IL-6, IL-8, IL-10, IL-18, and transforming growth factor β (TGFβ) (22).
This specific cytokine pattern seems to have a prognostic effect, since high IL-6 or IL-10 serum concentrations are associated with negative prognoses in independent cancer types whereas TNFα and IL-6 are recognized as master regulators of tumor-associated inflammation and tumourigenesis (23-25). Since there is a strong connection between IL-6 level and hepatic production of CRP, CRP level could be used as indirect measure of the tumor activity (26,27).
Elevated CRP level is already recognized as ominous prognostic factor in different malignancies including lung cancer (28-36). Among the tumor derived humoral factors, IL-6, IL-1 and macrophage colony stimulating factors are also related to pathophysiological mechanism of thrombocytosis and elevated fibrinogen level in cancer patients, which implies more complex relationship between cancer-related cytokine secretion and inflammation and coagulation process as well (11-13).
Different inflammation markers or their combinations prior treatment were assessed for their predictive value in lung cancer. Inflammation as predictive factor, was analyzed in resectable NSCLC by Alifano et al., who found that inflammation with nutrition, and tumoral immune contexture may predict the outcome (37). Cedrés et al. found a direct association between a high neutrophil to lymphocyte ratio NLR value as marker of systemic inflammation and poor prognosis in NSCLC patients, and this was similarly observed by Botta et al. in NSCLC patients treated with bevacizumab (38,39). Kasymjanova et al. combined CRP and white blood cells (WBCs) in prognostic index and found that it can be a prediction tool for treatment and survival in metastatic NSCLC (40).
In this study predictive value of non-infectious inflammation marker CRP in interaction with fibrinogen on the chemotherapy response was analyzed. We found the relationship between lung cancer on the one side and fibrinogen and the CRP level as inflammatory and coagulation indirect markers on the other. The significant correlation of elevated pretreatment CRP level with poorer outcome after given chemotherapy was registered. There was a statistically significant difference in the pretreatment CRP level between the patients with disease control or progression at re-evaluation. The group of patients who had high CRP levels and the low fibrinogen level had poorer response to the chemotherapy with higher probability of disease progression at re-evaluation. The patients, who had low CRP levels before the start of the treatment, responded better to the frontline chemotherapy. Additionally, we found the statistically significant difference in dynamics in CRP and fibrinogen level pre- and post-treatment in these two groups of patients.
These findings are suggestive for complex interaction between cancer, non-infectious inflammation and coagulation cascade. Our study has several limitations: it was designed as retrospective study; patients in the study were with good PS only, neutrophil to leukocyte count were not included in analysis, treatment response was assessed by two radiological methods. Still, the results from our study support the value of elevated pre-treatment CRP value in prediction of the first line treatment outcome. Findings from this study contribute the growing evidence of inflammation and cancer relationship, with negative predictive impact of existing pre-treatment non-infectious inflammation on the NSCLC frontline chemotherapy response. Therefore, CRP and fibrinogen levels could be used as adjacent predictive tool in deciding on therapeutic strategy for patients with locally advanced and metastatic disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by UHC Zagreb Ethics Committee and University of Zagreb Medical School Faculty Board (No. 380-59-10106-13-51/13) and written informed consent was obtained from all patients.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [Crossref] [PubMed]
- Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest 2002;122:1037-57. [Crossref] [PubMed]
- Jeremic B, Milicic B, Dagovic A, et al. Pretreatment clinical prognostic factors in patients with stage IV non-small cell lung cancer (NSCLC) treated with chemotherapy. J Cancer Res Clin Oncol 2003;129:114-22. [PubMed]
- Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii56-64. [Crossref] [PubMed]
- Azzoli CG, Temin S, Giaccone G. 2011 focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Oncol Pract 2012;8:63-6. [Crossref] [PubMed]
- Scott HR, McMillan DC, Forrest LM, et al. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer 2002;87:264-7. [Crossref] [PubMed]
- Moore MM, Chua W, Charles KA, et al. Inflammation and cancer: causes and consequences. Clin Pharmacol Ther 2010;87:504-8. [Crossref] [PubMed]
- Ding YP, Feng BY, Ma ZF. Levels of VIIIR:Ag, VIII:C and fibrinogen in plasma of patients with lung cancer. Zhonghua Jie He He Hu Xi Za Zhi 1994;17:301-2, 319-20.
- Pedersen LM, Milman N. The prognostic value of thrombocytosis in patients with primary lung cancer. Ugeskr Laeger 1998;160:3917-20. [PubMed]
- Kimura H, Ishibashi T, Shikama Y, et al. Interleukin-1 beta (IL-1 beta) induces thrombocytosis in mice: possible implication of IL-6. Blood 1990;76:2493-500. [PubMed]
- Suzuki M, Ohwada M, Aida I, et al. Macrophage colony-stimulating factor enhances platelet recovery following cisplatin/carboplatin chemotherapy in ovarian cancer. Gynecol Oncol 1994;54:23-6. [Crossref] [PubMed]
- Leven RM, Clark B, Tablin F. Effect of recombinant interleukin-6 and thrombopoietin on isolated guinea pig bone marrow megakaryocyte protein phosphorylation and proplatelet formation. Blood Cells Mol Dis 1997;23:252-68. [Crossref] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods 2009;41:924-36. [Crossref] [PubMed]
- DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev 2010;29:309-16. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39-51. [Crossref] [PubMed]
- Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073-81. [Crossref] [PubMed]
- Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;449:557-63. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013;14:e218-28. [Crossref] [PubMed]
- Galizia G, Orditura M, Romano C, et al. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol 2002;102:169-78. [Crossref] [PubMed]
- Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci 2012;8:1248-53. [Crossref] [PubMed]
- Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis 2011;70 Suppl 1:i104-8. [Crossref] [PubMed]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448-54. [Crossref] [PubMed]
- McKeown DJ, Brown DJ, Kelly A, et al. The relationship between circulating concentrations of C-reactive protein, inflammatory cytokines and cytokine receptors in patients with non-small-cell lung cancer. Br J Cancer 2004;91:1993-5. [Crossref] [PubMed]
- Casamassima A, Picciariello M, Quaranta M, et al. C-reactive protein: a biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 based immunotherapy. J Urol 2005;173:52-5. [Crossref] [PubMed]
- Kelly L, White S, Stone PC. The B12/CRP index as a simple prognostic indicator in patients with advanced cancer: a confirmatory study. Ann Oncol 2007;18:1395-9. [Crossref] [PubMed]
- Geissbühler P, Mermillod B, Rapin CH. Elevated serum vitamin B12 levels associated with CRP as a predictive factor of mortality in palliative care cancer patients: a prospective study over five years. J Pain Symptom Manage 2000;20:93-103. [Crossref] [PubMed]
- Guillem P, Triboulet JP. Elevated serum levels of C-reactive protein are indicative of a poor prognosis in patients with esophageal cancer. Dis Esophagus 2005;18:146-50. [Crossref] [PubMed]
- Karakiewicz PI, Hutterer GC, Trinh QD, et al. C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer 2007;110:1241-7. [Crossref] [PubMed]
- Beer TM, Lalani AS, Lee S, et al. C-reactive protein as a prognostic marker for men with androgen-independent prostate cancer: results from the ASCENT trial. Cancer 2008;112:2377-83. [Crossref] [PubMed]
- Suh SY, Ahn HY. A prospective study on C-reactive protein as a prognostic factor for survival time of terminally ill cancer patients. Support Care Cancer 2007;15:613-20. [Crossref] [PubMed]
- Hara M, Matsuzaki Y, Shimuzu T, et al. Preoperative serum C-reactive protein level in non-small cell lung cancer. Anticancer Res 2007;27:3001-4. [PubMed]
- Liao C, Yu Z, Guo W, et al. Prognostic value of circulating inflammatory factors in non-small cell lung cancer: a systematic review and meta-analysis. Cancer Biomark 2014;14:469-81. [PubMed]
- Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One 2014;9:e106914. [Crossref] [PubMed]
- Cedrés S, Torrejon D, Martínez A, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol 2012;14:864-9. [Crossref] [PubMed]
- Botta C, Barbieri V, Ciliberto D, et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther 2013;14:469-75. [Crossref] [PubMed]
- Kasymjanova G, MacDonald N, Agulnik JS, et al. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol 2010;17:52-8. [Crossref] [PubMed]


