Evaluation of a disease management program for COPD using propensity matched control group
Introduction
Chronic obstructive pulmonary disease (COPD) is a growing health concern causing chronic morbidity and mortality worldwide (1). The prevalence of moderate to severe COPD in Singapore is estimated at 2.3% (2), or absolute number of 87,819 patients in the community. These patients have frequent hospital admissions (3), and emergency department attendances (4), costing the country about US$128 million a year (5). In 2010, COPD was ranked as the seventh leading cause of death and hospitalization in Singapore, accounting for 2.5% of total deaths and more than 10,000 admissions (6). These numbers are expected to grow with increasing prevalence of smoking, thus burdening health care services in Singapore (7).
Disease management programs (DMPs) have proliferated recently as a means of improving the quality and efficiency of care for patients with chronic illness (8). These programs include education about disease, optimization of evidence-based medications, information and support from case managers, and institution of self-management principles (9). Evidence exists on successful implementation of DMPs for chronic conditions such as COPD, heart failure and diabetes mellitus (10). However, with the introduction of DMP against a background of resource constraints in the healthcare sector, policymakers, healthcare managers, planners and funders increasingly want to know if implementing such approaches improve the quality and reducing the cost of healthcare, and, ultimately, improving health outcomes for the chronically ill.
Evaluation of healthcare programs and interventions has become an important component of decision making on the (public) funding of new health technologies and wider dissemination of proven health interventions. The randomized controlled trial is considered the gold-standard research and program evaluation design (11,12); however, randomized control trial (RCT) design may not be suitable for many research endeavours unless the study is conducted in a tightly controlled environment. Disease management (DM) by its very nature is population based and, thus cannot be tightly controlled. The most common method currently used in DM evaluation is referred to as a “total population approach” (13), a pre-test/post-test design which is a relatively weak research and evaluation technique (14-16). The most basic limitation of this design is that there is no randomized control group for which comparisons of outcomes can be made, thereby allowing several sources of bias and/or competing extraneous confounding factors to offer plausible alternative explanations from any change from baseline (13-16).
DM programs currently do not use randomized control groups under the belief that: (I) it would be costly and difficult to track behavioural change longitudinally and outcomes for a group not under their purview; (II) the organization may be hesitant to offer services to one subset of the population while withholding that same “value added” benefit to others; and (III) there is a need to treat all members with the disease (if these interventions are indeed clinically effective) because each member receiving the intervention has the potential of adding to the medical cost savings and positive clinical outcomes promised by the program. With these concerns in mind, we have considered an alternative approach, “propensity scoring”, which utilizes existing data sources to create randomly matched controls, which are conditional on having an adequate set of observable characteristics for both DMP participants and non-participants (17).
The National Healthcare Group’s (NHG) DMP for COPD was started in April 2008 to optimize resources for better management of patients afflicted with COPD and community acquired pneumonia (CAP) based on patient severity. The purpose of this study is to compare the outcomes of COPD patients enrolled in “DMP” with propensity matched controls who are not enrolled in the program.
Methods
Study design
This is a retrospective propensity matched cohort study comparing COPD patients (Diagnosis Related Group, DRG 177) who were enrolled in a DMP with controls who are COPD patients (DRG 177) who fulfilled the DMP enrolment criteria but were not enrolled (Figure 1). A control group was identified from Operations Data Store (ODS), they were COPD patients (DRG 177; ICD 490, 491, 492, 496) who fulfilled the DMP’s inclusion criteria but were not enrolled in the DMP. DMP patients were identified from Central Clinical Research Database (CCRD). The studies on “Disease management” are exempted from IRB approval (NHG DSRB) in our institutions; hence ethics approval was not obtained for this study.
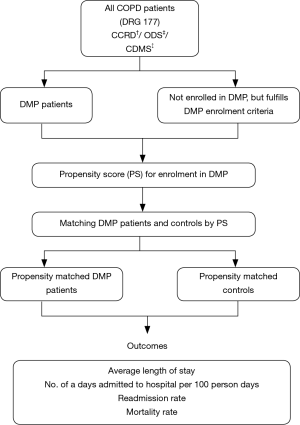
Hospitalizations and mortality in these patients were tracked from April 2008 to December 2009. Follow up time was from the first admission date to censor date (31 Dec 2009) or date of death and expressed as person years (PY). The outcomes of interest were average length of stay, number of days admitted to hospital per 100 person days, readmission and mortality rates per person year. Index admissions were the starting point for analysing repeat hospital visits. Readmissions were defined as any admission from the index admission. The 30-day readmissions include any admission within the 30-day window from the date of discharge of index admission.
Primary endpoints and data sources
The primary end points included all-cause mortality, mortality due to respiratory diseases (ICD-9 CM: 460–519), readmissions due to COPD (DRG 177; ICD 490, 491, 492, 496). Death data were obtained from Epidemiology and Disease Control division, Ministry of Health (MOH), Singapore. Hospitalization data for DMP patients and controls were obtained from CCRD and ODS administrative databases, respectively. Co-morbidity data was extracted from the Chronic Disease Management System (CDMS). Patients with hospitalization after enrolment/refusal were analysed.
Disease management program (DMP)
Disease management program (DMP) multi-disciplinary team
DMP consists of a core group of respiratory case managers who coordinate COPD care; they work collaboratively with the respiratory physicians, ED physicians and general practitioners to manage both conditions across the continuum. DMP follows the long accepted principles of chronic DM such as stratification of patients by the acuity of disease, evidence-based algorithms, fast track protocols to specialist outpatient clinics (SOCs), team-based approach, and self-management.
Disease management program (DMP) core components
Besides the conventional COPD care which included pharmacological interventions, a comprehensive, individualized patient education for self-management of COPD is conducted to prevent exacerbations, to reduce unnecessary re-admissions and to improve quality of life. Telephonic case management is done for all patients to ensure compliance to clinic appointments (usually 3–4 months), medications and thereby treatment optimization. The home care plan is provided for selected patients during their post-discharge period. For patients who are stable and are capable of self-management, the case manager will call the patients once every 2 months. Patients who are discharged after an acute exacerbation of COPD were monitored more closely with telephone calls once a week. Smoking cessation, rehabilitation and vaccination programs were enhanced for DMP patients. Control patients had conventional pharmacological interventions.
Disease management program (DMP) inclusion and exclusion criteria
Inclusion criteria for DMP were patients with definite COPD [forced expiratory volume in 1 second (FEV1)/forced volume capacity (FVC) <70%] or possible COPD based on clinical judgment, patients should be willing to participate in the program, comply with telephonic case management and medical instructions, willing to attend a smoking cessation counselling program and attempt to quit smoking. Patients who do not fulfill the diagnosis of COPD, patients with complicated medical conditions (e.g., advanced malignancy); patients who are clinically unstable requiring mechanical ventilation, intensive care support, patients with cognitive, psychiatric disorders were excluded from DMP.
Disease management program (DMP) recruitment
At the emergency department all diagnosed COPD patients are evaluated for clinical severity, high risk and intermediate risk patients are hospitalized, while low risk patients are evaluated for home care and given an outpatient appointment in 2 weeks, a telephone contact and a home visit, where appropriate follow. If the home care plan fails, the patient is transferred back to the hospital.
Principles of propensity scoring
In general, DMPs provide high-intensity interventions (such as telephonic nurse/education services) to only a small number of participants out of a much larger population of patients with similar disease. Matched sampling techniques attempt to choose members from the untreated population so that they are similar to the program participants with respect to one or more pre-program variables. Controlling for differences in pre-intervention characteristics is extremely important in DMP because program participants are typically dissimilar to non-participants (as a rule, DM program strives to enrol those individuals at the highest risk for incurring higher costs or higher healthcare utilization during the program term, thereby creating an unbalanced case mix between enrolled and non-enrolled groups. The propensity score, defined as the probability of assignment to the treatment group, conditional on covariates (i.e., independent variables) (18), can control for pre-intervention differences between the enrolled and non-enrolled groups, with the underlying assumption that DMP is associated with observable pre-program variables (e.g., age, sex, utilization, and cost) (19,20). Propensity scores are derived from a logistic regression equation, which reduced each participant’s set of covariates into a single score, making it feasible to match on what are essentially multiple variables simultaneously (16).
Estimation of propensity scores and matching
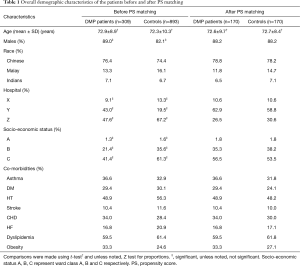
We used propensity score matching to adjust for differences in baseline characteristics between DMP patients and controls (21,22). Propensity scores for DMP enrolment using a non-parsimonious multivariable logistic regression model which included the following variables, age, gender, race, hospital, Socio-economic status, comorbidities: presence of asthma, diabetes mellitus, hypertension, stroke, coronary heart disease, heart failure, dyslipidaemia and obesity. Ward class was used as a surrogate for socio-economic status (SES). Our propensity score model discriminated well between DMP patients and controls. (C-statistic =0.79). We then used the propensity score to match each DMP patient to a control, who had a similar propensity score using nearest neighbour without the replacement matching algorithm, thus matching 171 DMP patients to 171 controls.
Statistical analysis
Demographic, health care utilization characteristics were assessed as counts and percentages for categorical variables, and as standard measures (mean and SD) for continuous variables. We used Chi-square tests, independent sample t-tests and paired t-tests, as appropriate, for descriptive analysis to compare baseline characteristics between pre-match DMP patients and controls. For descriptive analysis of post-match cohorts, McNemar tests were used. Adequacy of matching was assessed using P values for comparison tests and standardized percentage differences (21,22).
Mortality and readmission rates were calculated by dividing the number of events during follow-up by the corresponding PY at risk. All rates were presented as number of events per PY with corresponding 95% confidence intervals (CI). We used Kaplan-Meier survival analyses, matched Cox proportional hazards model and competing risk regression (23,24), to estimate the associations between mortality, morbidity (hospitalizations and visits), and participation in DMP. We confirmed proportional hazards assumption by a visual examination of the log (minus log) curves. All significance tests were two-tailed and data analyses were performed with the STATA 11.2 software (StataCorp, College Station, TX).
Results
Overall there were 334 DMP and 893 control patients with 488 and 1,227 hospitalizations respectively. Median follow up period was 0.99 & 0.66 years for DMP and control patients respectively. Total PYs of follow-up was higher for controls (739 PY) when compared to DMP patients (269 PY).
Demographic and admission characteristics
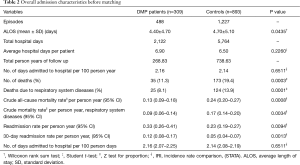
Table 1 summarizes the demographic characteristics of all patients. There were significant differences between DMP patients and controls in gender, hospitals and SES categories (Table 1). DMP patients had significantly shorter length of stay at the hospital, lower percentage of all-cause deaths and deaths due to respiratory system diseases. All-cause mortality rate per person year and mortality rate due to respiratory system diseases was lower for DMP patients. Readmission rate per person year and 30-day readmission rate was significantly higher for DMP patients (Table 2).
Full table
Full table
Propensity score computation and matching
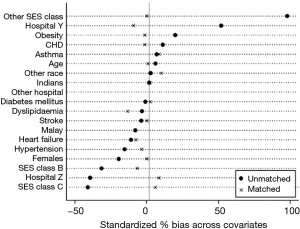
DMP patients were matched to controls by their propensity to be enrolled in the program. Odds for enrolment in DMP was higher for patients from hospital B [odds ratio (OR) =2.5; 95% CI, 1.5–4.3] and patients with obesity (OR =1.7; 95% CI, 1.2–2.3) and lower for patients with hypertension (OR =0.58; 95% CI, 0.42–0.80). The performance of the prediction model was evaluated by receiver operating characteristic curve (ROC) analysis, C-statistic was 0.79 indicating good predictive power—variables selected in our propensity model were highly predictive of the treatment (in this case, enrolment in the DMP). After matching, the socio-demographic characteristics and comorbidities between the DMP and controls were similar (Table 1). Figure 2 shows the covariate balance before and after PS matching; the standardized bias percentage has reduced considerably across the covariates.
Demographic and admission characteristics after matching
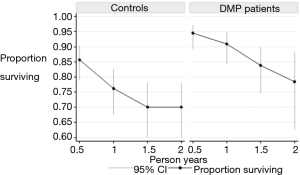
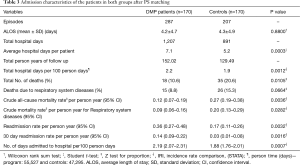
Table 3 shows the matched DMP patients and controls. The 170 matched DMP patients and control patients had 287 and 207 hospitalizations respectively. Median follow up period was 1.1 and 0.62 years for the matched DMP and control patients respectively. DMP patients and controls were similar with regards to age, gender, race, hospital, SES, comorbidities and average hospital length of stay (Tables 1,3). DMP patients had lower mortality than the controls (0.12 vs. 0.27 per person year); cumulative 1-year survival was 91% and 76% between DMP and control patients respectively (Figure 3). Hospital days per 100 person-days were higher for DMP patients (2.19 vs. 1.88) (Table 3).
Full table
Risk of death and readmission
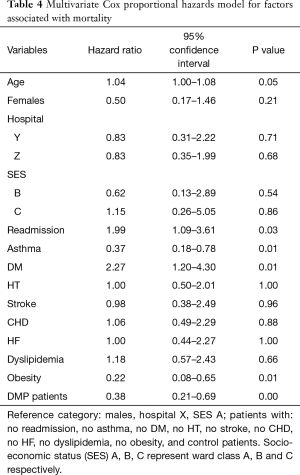
The risk of death was lower in DMP patients [hazard ratio (HR) =0.38; 95% CI, 0.21–0.69], patients with asthma (HR =0.37; 95% CI, 0.18–0.78), obesity (HR =0.22; 95% CI, 0.08–0.65) and higher for older patients (HR =1.04; 95% CI, 1.00–1.08) and patients with DM (HR =2.3; 95% CI, 1.2–4.3) (Table 4). Risk of readmission was higher for DMP patients than control patients (adjusted for competing risk—mortality, SHR =3.4; 95% CI, 1.8–6.4) (Table 5).
Full table

Full table
Discussion
In this retrospective propensity matched cohort study of DMP patients and controls, participation in DMP had reduced 1-year all-cause mortality by 15%. However, DMP patients had higher readmissions and total hospital days, the survival gain may have contributed to this increase. To our knowledge, this is the first comprehensive DMP for COPD to show mortality reduction, other studies of pharmacological interventions showed similar reductions in mortality (25).
Literature shows that smoking cessation has been the single most effective and cost-effective way to reduce the risk of developing COPD and stop its progression (26). In this study, DMP patients were enrolled in a smoking cessation program and counselled on quitting smoking by the case managers, the counselling and advice were sustained through telephonic case management. Studies worldwide have shown that even in severe COPD, smoking cessation slows the accelerated rate of lung function decline and improves survival compared with continued smoking (27,28). A recent study of smoking cessation and COPD mortality among Japanese men and women, shows smoking cessation reverses the excess risk of COPD mortality to a level similar to that observed among never smokers in men (29). These data suggest that the inflammatory changes are reversible rapidly after smoking cessation and could explain the lower risk of death observed among DMP patients. Other components of DMP such as timely follow up with the physicians coordinated by a case manager, telephonic case management, optimization of medications, self-management of the condition and timely treatment for exacerbation at the hospital would have had a synergistic effect on survival.
Previous trials and systematic reviews of DM in COPD were heterogeneous in terms of size, multi-component interventions, duration of follow up and outcomes. These studies have reached differing conclusions (30), majority of the studies had recognized the potential value of this type of intervention for reducing hospital readmissions and ED attendances (31). However, in a RCT, comparing a comprehensive care management program (CCMP) with usual care for COPD, CCMP in patients with severe COPD had not decreased COPD-related hospitalizations (32). In another multicentre RCT, among patients with diabetes, COPD, and congestive heart failure, the intervention designed to improve patients’ access to primary care providers, the coordination of outpatient services, and the provision of comprehensive and continuous care (program components similar to DMP) increased the rate of rehospitalisation (33).
Mortality or readmission with 30 days suggests the possibility of inadequate care (34). However growing evidence reveals that mortality and readmissions may in fact be inversely associated with one another (35,36). In our study, DMP patients had lower mortality but higher readmission than controls. The results are different from similar studies elsewhere (37). Our findings suggest that readmissions could be “adversely” affected by a competing risk of death. A patient who dies during the index episode of care can never be readmitted. Since DMP patients have low mortality, a greater proportion of discharged DMP patients are eligible for readmission. As such, a higher readmission rate may be a consequence of successful care in DMP. Studies show, case management improved access to resources and staff-patient communication (38). Heightened monitoring of discharged patients in a DMP provides a channel for patient to voice their complaints resulting in more readmissions but also saving more lives (39). Six percent of DMP patients had over 4 readmissions (frequent flyers) and accounted for 42% of total readmissions in comparison there were none in the control group who had more than four readmissions. The higher readmissions among DMP patients are partially driven by the frequent flyers.
Hospitalization for exacerbation of COPD represents a very complex, multi-factorial picture and is associated with high risk of mortality (40). Vast majority these readmissions could be attributable to “unavoidable” issues such as disease progression or social factors (8). Furthermore, preventing or mitigating exacerbation in COPD is essential to prolonging life, this would require hospitalization for at least 35% of the patients and may lead to readmission (41). Therefore, DMP planning for COPD should take into account the inverse relationship between readmission and mortality.
This study has several strengths. It has led to a greater understanding of the local COPD population and how a CCMP affects outcomes for patients enrolled in the program. The study had complete mortality follow-up and provides estimates of the adjusted sub-hazard of COPD readmission using Fine and Gray competing risk framework. The estimated risks of mortality and readmission are more reliable and less likely to be biased. Some of the limitations are, first, since the evaluation focuses on patients who had hospitalization (severe patients), the results may not be generalizable to all COPD patients. This analysis has been adjusted for demographics and comorbidities and not adjusted for lung function and disease severity. Second, we did not report spirometry which would be useful as it is the gold standard for COPD classification. However, our study was a real-world experiment conducted using administrative database, and spirometry was not performed for everyone, hence we were not able to report this. Third, readmissions were captured only for the three hospitals, admissions/readmissions that happened outside these three hospitals are not captured. Lastly this evaluation has established associations, not causality.
The DMP has led to improved survival and added years of life to patients with COPD. However readmission rates were higher among DMP patients, this could be due to increased patient contact with the system facilitated by the case managers and could be partially due to frequent flyers (patients with four or more admissions). Increase in readmission may lead to hospital bed crunch, choking up the already scarce healthcare resources at the hospital. This calls for better coordination of COPD services with primary, intermediate and long-term care partners.
In conclusion, the study highlights the usefulness of propensity scoring methodology for identifying appropriate control for evaluation of DMP from administrative data. Our analysis showed that participation in the “DMP” was associated with lower all-cause mortality and increased readmission. Planners have to be aware of the vicious cycle between readmission and death in severe ill COPD patients. Further studies are required to ensure corrections for case mix and time bias and unobserved confounders.
Acknowledgements
The authors would like to thank department colleagues Dr Joseph Molina for reviewing the manuscript and Dr. Ding Yew Yoong for his statistical advice.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The studies on “Disease management” are exempted from IRB approval (NHG DSRB) in our institutions, hence ethics approval was not obtained for this study.
References
- Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747-57. [Crossref] [PubMed]
- World Chronic Obstructive Pulmonary Disease (COPD) Day - the Singapore Perspective. The College Mirror 2003;29:18-9. Available online: http://www.cfps.org.sg/publications/the-college-mirror/download/48
- Cao Z, Ong KC, Eng P, et al. Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology 2006;11:188-95. [Crossref] [PubMed]
- Paul P, Heng BH, Seow E, et al. Predictors of frequent attenders of emergency department at an acute general hospital in Singapore. Emerg Med J 2010;27:843-8. [Crossref] [PubMed]
- Hattab Y, Alhassan S, Balaan M, et al. Chronic Obstructive Pulmonary Disease. Crit Care Nurs Q 2016;39:124-30. [Crossref] [PubMed]
- Address by Dr Amy Khor, Minister-of-State for Health at the Launch of COPD-ICP & Official Takeover of Jurong Medical Centre by JurongHealth on Saturday, 28 April 2012, 11.00 am at Jurong Medical Centre. Available online: https://www.moh.gov.sg/content/moh_web/home/pressRoom/speeches_d/2012/address_by_dr_amykhorminister-of-stateforhealthatthelaunchofcopd.html
- Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523-32. [Crossref] [PubMed]
- Weingarten SR, Henning JM, Badamgarav E, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ 2002;325:925. [Crossref] [PubMed]
- Hunter DJ, Fairfield G. Disease management. BMJ 1997;315:50-3. [Crossref] [PubMed]
- Glasgow RE, Funnell MM, Bonomi AE, et al. Self-management aspects of the improving chronic illness care breakthrough series: implementation with diabetes and heart failure teams. Ann Behav Med 2002;24:80-7. [Crossref] [PubMed]
- D’Arcy Hart P. Early controlled clinical trials (letter to the Editor). Br Med J 1996;312:378-9. [Crossref]
- Maynard A, Chalmers I, editors. Non-random reflections on health services research: On the 25th Anniversary of Archie Cochrane's Effectiveness and Efficiency. 1st Edition. London: BMJ Books; 1997.
- Linden A, Adams JL, Roberts N. An assessment of the total population approach for evaluating disease management program effectiveness. Dis Manag 2003;6:93-102. [Crossref] [PubMed]
- Campbell DT, Stanley JC. Experimental and quasi-experimental designs for research. Boston: Houghton Mifflin; 1963.
- Cook TD, Campbell DT. Quasi-experimentation: design & analysis issues for field settings. Boston: Houghton Mifflin; 1979.
- Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Boston: Houghton Mifflin; 2002.
- Linden A, Adams JL, Roberts N. Using propensity scores to construct comparable control groups for disease management program evaluation. Disease Management & Health Outcomes 2005;13:107-15. [Crossref]
- Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat 2002;84:151-61. [Crossref]
- Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. J Educ Psychol 1974;66:688-701. [Crossref]
- Rubin DB. Assignment to treatment group on the basis of a covariate. J Educ Behav Stat 1977;2:1-26. [Crossref]
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [Crossref] [PubMed]
- Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistician 1985;39:33-8.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 1999;94:496-509. [Crossref]
- Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389-430. [Crossref] [PubMed]
- Sin DD, Man SF. Pharmacotherapy for mortality reduction in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:624-9. [Crossref] [PubMed]
- Faulkner MA, Lenz TL, Stading JA. Cost-effectiveness of smoking cessation and the implications for COPD. Int J Chron Obstruct Pulmon Dis 2006;1:279-87. [Crossref] [PubMed]
- Godtfredsen NS, Lam TH, Hansel TT, et al. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Respir J 2008;32:844-53. [Crossref] [PubMed]
- Hersh CP, DeMeo DL, Al-Ansari E, et al. Predictors of survival in severe, early onset COPD. Chest 2004;126:1443-51. [Crossref] [PubMed]
- Li Y, Yamagishi K, Yatsuya H, et al. Smoking cessation and COPD mortality among Japanese men and women: the JACC study. Prev Med 2012;55:639-43. [Crossref] [PubMed]
- Ofman JJ, Badamgarav E, Henning JM, et al. Does disease management improve clinical and economic outcomes in patients with chronic diseases? A systematic review. Am J Med 2004;117:182-92. [Crossref] [PubMed]
- Peytremann-Bridevaux I, Staeger P, Bridevaux PO, et al. Effectiveness of chronic obstructive pulmonary disease-management programs: systematic review and meta-analysis. Am J Med 2008;121:433-443.e4. [Crossref] [PubMed]
- Fan VS, Gaziano JM, Lew R, et al. A comprehensive care management program to prevent chronic obstructive pulmonary disease hospitalizations: a randomized, controlled trial. Ann Intern Med 2012;156:673-83. [Crossref] [PubMed]
- Weinberger M, Oddone EZ, Henderson WG. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med 1996;334:1441-7. [Crossref] [PubMed]
- Ashton CM, Del Junco DJ, Souchek J, et al. The association between the quality of inpatient care and early readmission: a meta-analysis of the evidence. Med Care 1997;35:1044-59. [Crossref] [PubMed]
- Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes 2010;3:459-67. [Crossref] [PubMed]
- Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? N Engl J Med 2010;363:297-8. [Crossref] [PubMed]
- Egan E, Clavarino A, Burridge L, et al. A randomized control trial of nursing-based case management for patients with chronic obstructive pulmonary disease. Lippincotts Case Manag 2002;7:170-9. [Crossref] [PubMed]
- O'Connor CM, Fiuzat M. Is rehospitalization after heart failure admission a marker of poor quality? Time for re-evaluation. J Am Coll Cardiol 2010;56:369-71. [Crossref] [PubMed]
- Ulrik CS. No benefit and potential harm with an educational and care management programme for chronic obstructive pulmonary disease. Evid Based Med 2013;18:72-3. [Crossref] [PubMed]
- van Walraven C, Bennett C, Jennings A, et al. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011;183:E391-402. [Crossref] [PubMed]
- Suissa S, Dell'Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012;67:957-63. [Crossref] [PubMed]