Robotic-assisted thoracoscopic sleeve lobectomy for locally advanced lung cancer
Introduction
In the past two decades, several studies have demonstrated the advantages of shorter hospital stay, less tissue injury, better cosmetic results, lower morbidity and equivalent oncologic results with video-assisted thoracoscopic (VATS) lobectomy compared with open thoracotomy for lung cancer surgery (1-3). While thoracoscopic surgery has long been considered a standard procedure, robotic surgery is gaining popularity. Melfi et al. reported the first experience of robotic-assisted thoracoscopic surgery (RATS) for lung cancer in 2002 (4). Thereafter, RATS has been proposed as an alternative choice in the lung cancer surgery field. There are several practical advantages of RATS over VATS in lung cancer surgery, including its provision of a three-dimensional field of vision and of greater articulation with endowrist technology. On the other hand, opponents of robotic surgery have cited the expensive cost, loss of haptic feedback, longer procedure times and concern regarding the management of accidental intraoperative bleeding (5-7). The impact of RATS lobectomy on clinical outcomes remains unclear. A few studies have reported on early experiences of using RATS for lung resection, and all have indicated similar outcomes in comparison with using VATS (5-7). Potential benefits may be postoperative pain reduction, early return to usual activity (5), and fewer conversions for uncontrolled bleeding (7).
Compared with standard lobectomy or wedge resection, the surgical technique of sleeve lobectomy is more complex, which has limited the usage of minimally invasive surgery in performing the procedure. VATS sleeve lobectomy or bronchoplasty has been reported, but its suitability is limited (8,9). The RATS platform may be superior to VATS for such complex thoracic surgeries because of its three-dimensional viewing camera and articulated robotic forceps. However, there have been only three studies on the use of RATS for performing bronchoplasty or sleeve lobectomy (10-12). Here, we report our early experience conducting six RATS sleeve lobectomies for locally advanced lung cancer. This is the first series report of RATS sleeve lobectomy for lung cancer in the current English literature.
Methods
Patient population
The six consecutive NSCLC patients who underwent RATS sleeve lobectomy from November 2013 to July 2015 at National Taiwan University Hospital, all performed by a single surgeon (JM Lee), were enrolled in this study. Sleeve resection was considered as the first option in all patients with centrally located NSCLC if it was possible to perform complete resection that would result in sufficient spared lung parenchyma to occupy the thoracic cavity. We started to use the RATS technique for performing sleeve lobectomies beginning in 2013 and henceforth recommended RATS for all candidates of sleeve lobectomy. However, the choice between RATS and VATS was mainly decided by the patients themselves due to the high cost of RATS. On average, the in-hospital cost of RATS is around 6,000 USD more expensive than VATS in our institute. The preoperative staging procedure incorporated chest radiography; blood chemistry analysis and serum carcinoembryonic antigen measurement; computed tomography of the chest, brain, and abdomen; positron emission tomography scans or bone scans; bronchoscopy; echocardiogram; and pulmonary function tests. Clinicopathologic and perioperative parameters as well as postoperative outcomes were collected retrospectively from chart review. The study was approved by the National Taiwan University Hospital Research Ethics Committee, and all patients signed an informed consent.
Surgeon background
Professor JM Lee is the chief in the Thoracic Division of National Taiwan University Hospital. He has performed more than 2,000 previous VATS lobectomies, and his previous robotic experience includes more than 150 cases of RATS lobectomy, thymectomy, esophagectomy and mediastinal tumor resection.
Robotic-assisted thoracoscopic sleeve lobectomy
The patients were placed in the right or left decubitus position, according to the lesion site, and the three robotic arms (da Vinci Surgical System, Intuitive Surgical Inc., Sunnyvale, CA, USA) were used in the routine manner for RATS in our hospital. For the 3-arm setting, the port sites were slightly more lateral as compared to that created in the 4-arm setting. A utility incision was made on the tip of the 9th or 10th rib on the internal edge of diaphragm. The 30-degree downscope was introduced through the port created along the 7th intercostal space on the mid-axillary line. The first and second robotic arms were introduced through the ports located on 5th to 6th intercostal space and 8th to 9th intercostal space on the anterior or posterior axillary line respectively, the location of which was determined by a scope introduced through the utility incision to ensure the location of the ports was just above the major fissure. The assistant was standing in front of the patient working through the utility incision port.
In the dissection phase, the unipolar electrocautery instrument (Permanent Cautery Spatula, Intuitive Surgical Inc., Sunnyvale, CA, USA) was in the right robotic arm and the bipolar electrocautery instrument (Fenestrated Bipolar Forceps, Intuitive Surgical Inc., Sunnyvale, CA, USA) was in the left robotic arm. The main pulmonary vein, artery and bronchus were divided with a linear stapler by the assistant through the anterior utility-incision port without dislodging any of the robotic arms.
For bronchial anastomosis during the sleeve lobectomy, a needle driver was introduced to replace the electrocautery instrument. Intraoperative pathological frozen-section analysis was routinely performed to confirm the negativity of the bronchial or vascular stump involvement by cancer cells in all patients. End-to-end bronchial anastomosis was accomplished by a continuous running suture with 4-0 absorbable monofilament stitches (PDSII, Ethicon Inc., Somerville, NJ, USA) (Figure 1). Bronchoscopy was used to confirm the integrity of the anastomosis site thereafter. All patients underwent systematic mediastinal and hilar lymph node dissection. After the surgery, lungs were checked for air leaks by inflation. A 28-French chest tube was placed through the anterior axillary line working port.
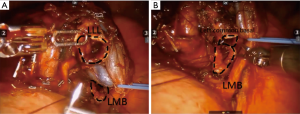
Results
Of the 6 study patients, 4 were male and 2 were female. The mean age of the patients was 58.3 years (range, 44–75). Two third of the patients were smokers (66.6%; 4/6). The pathologic diagnosis was squamous cell carcinoma in 5 of the patients, and carcinoid tumor in the remaining patient. The pathologic staging was IB in 3 patients, IIB in 2 and IIIA in 1 patient. The details of the preoperative demographic data and clinical parameters are listed in Table 1.

Full table
All patients underwent RATS sleeve lobectomy successfully without any conversion to open thoracotomy. The mean operative time was 436.7 min (range, 255–745). The number of lymph nodes dissected was 15.7 (range, 5–35). A large amount of intraoperative bleeding was noted in two patients (1,600 and 2,400 cc). The mean intensive care unit (ICU) stay and hospital stay were 3.7 (range, 1–11) and 11.3 (range, 3–26) days, respectively. Postoperative morbidity occurred in 2 patients (2/6, 33.3%), anastomosis stenosis in one and pneumonia in another. Postoperative cancer recurrence was noted in one patient (1/6, 16.7%). The perioperative parameters and postoperative outcomes are listed in Table 2. Figure 2 illustrates preoperative and postoperative computed tomography, and shows the patent bronchial anastomosis under computed tomography imaging and bronchoscopy after surgery.

Full table
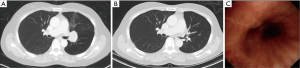
Discussion
With the advances made in VATS in the 1990s, early stage lung cancer surgeries are increasingly done by VATS lobectomy because it results in shorter hospital stays, less tissue injury, and better cosmetic results than open lobectomy. In recent years, uniportal VATS for major lung resections has become a revolution in the treatment of lung cancers. More complicated procedures including uniportal segmentectomy, pneumonectomy, and sleeve resection have been reported (13,14). However, the application of VATS for locally advanced, centrally located lung cancers remains controversial. VATS sleeve lobectomy or bronchoplasty for locally advanced lung cancers has been sporadically reported (8,9), but its feasibility is limited because of its limited field of vision and maneuverability of instrumentation. In contrast, robotic surgery does allow for clear three-dimensional vision of the surgical field and provides ample maneuverability with its highly articulated robotic forceps. Therefore, RATS sleeve lobectomy or bronchoplasty for locally advanced lung cancers may be a better choice than a VATS procedure.
In 2006, Ishikawa et al. performed the first RATS sleeve lobectomy in a human cadaver (15). In actual clinical patients, only three cases have been reported in the English literature (10-12). The first report of robotic-assisted sleeve lobectomy was from Schmid and colleagues in 2011 (10). They performed a right upper lobe sleeve lobectomy for a typical carcinoid using hybrid surgery, including RATS for airway reconstruction and VATS for the other procedures. The postoperative course was uneventful, and the patient was discharged on postoperative day 15. Subsequently, Nakamura and colleagues performed robotic bronchoplastic right upper lobectomy for centrally located squamous cell carcinoma in 2012 (11). In 2015, Pan et al. reported their experience with extended sleeve lobectomy, a left lower and lingual lobectomy for a lung adenocarcinoma patient receiving neoadjuvant chemotherapy (12). In the current study, we reported the first series of six consecutive RATS sleeve lobectomies in our single institution. No conversion to thoracotomy and no mortality were noted.
Compared with VATS lobectomy, a longer operation time in RATS lobectomy has been reported previously (161–269.4 vs. 128–253.8 minutes) (7,16). The large range of operation times among the different studies may be related to differences in the amount of experience performing the procedure of the surgeons and institutes, differences in the definition of operation time (including the time spent on set-up, duration of the bronchoscopy exam and whether intraoperative frozen section for pathology study was done or not). In this study, the mean operation time was 436.7 (range, 255–745) min. A long operation time of more than 5 hours was noted in three patients (50%; 3/6). We suppose the prolonged operation time for these three patients was related to their having undergone preoperative neoadjuvant chemotherapy, a redo procedure, and a more complex procedure of double sleeve lobectomy with bronchus and vascular anastomosis. Besides, a large amount of intraoperative bleeding was noted in two of them. Undergoing preoperative neoadjuvant chemotherapy treatment or a redo procedure could make surgery more difficult by increasing pleural space adhesion and fibrosis of perivascular lymph nodes. However, in these three patients, the postoperative course and hospital stay were similar to those of the other group. Nakanishi reported his early experience of performing VATS sleeve lobectomy and bronchoplasty on five patients in 2007 (8). The mean operation time was 552.4 (range, 330–740) minutes. Mahtabifard and his colleagues reported another series of 13 VATS sleeve lobectomies in 2008 (9), with a mean operation time of 167 (range, 90–300) minutes. Our experience suggests that RATS is a feasible procedure with an equivalent operation time and may offer some advantages over conventional thoracoscopic surgery in such difficult situations.
Common complications after sleeve lobectomy include pneumonia, bronchial stenosis, atelectasis, prolonged air leakage, atrial fibrillation and wound infection, and the reported incidence of complication ranges from about 24–40% and 10–31% in open thoracotomy and VATS, respectively (9,17,18). The variability of complication rates among different studies may depend on their different studied populations and postoperative management (9,17,18). In the current study, two (33%; 2/6) postoperative morbidities occurred, including one of anastomosis stenosis and the other of postoperative pneumonia. The complication rate was equivalent to that reported in other series (9,17,18). Both of the two patients with postoperative morbidity had comorbidities, including diabetes and end-stage renal disease with regular hemodialysis. For the two patients with postoperative morbidities, the ICU stay and hospital stay were longer than those without postoperative morbidities (8 vs. 1.5 and 22.5 vs. 5.75 days, respectively).
Our previous thoracic robotic experience consists of more than 150 lobectomies, thymectomies, esophagectomies and other cases (19,20). This experience allowed successful performance of such a delicate procedure. Our experience suggests that there are several limitations of VATS sleeve lobectomy: (I) the usage of a traditional endoscopic needle holder is not convenient for suturing and knot tying; (II) the articulation of the manual endoscopic suture is limited; (III) the view afforded by conventional thoracoscopic surgery is especially limited in the suturing of the membranous portion, which is located at the bottom of the visual field. In contrast, the robotic system’s three-dimensional field of vision and the articulated joints of its instruments makes robotic-assisted bronchial anastomosis easier under the endoscopic setting. On the other hand, there are still some disadvantages of RATS surgery, including higher hospital costs and longer set-up times. Because this retrospective study involves a small case number of procedures performed in a single institute, more such procedures should be performed by multiple institutes to clarify the safety and feasibility of RATS sleeve lobectomy.
Conclusions
The operating time and complication rate of RATS sleeve lobectomy may be equivalent to conventional thoracoscopic surgery. Although RATS sleeve lobectomy is a complex technique, our experience showed its feasibility. Because RATS sleeve lobectomy is a relatively new procedure, both hospital cost and operating time may be decreased in the future as increasing familiarity with this procedure is gained. Further prospective randomized studies focusing on comparison of the feasibility and safety of RATS and VATS are necessary.
Acknowledgements
Funding: This study was supported by the Ministry of Science and Technology (MOST 104-2314-B-002-182-MY3), National Taiwan University Hospital (NTUH.105-S3005, HCH103-024), Taiwan Health Foundation, and Taiwan Society for the Chest Care of Taiwan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the National Taiwan University Hospital Research Ethics Committee and written informed consent was obtained from all patients.
References
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Paul S, Sedrakyan A, Chiu YL, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg 2013;43:813-7. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Louie BE, Farivar AS, Aye RW, et al. Early experience with robotic lung resection results in similar operative outcomes and morbidity when compared with matched video-assisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598-604; discussion 1604-5. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Nakanishi K. Video-assisted thoracic surgery lobectomy with bronchoplasty for lung cancer: initial experience and techniques. Ann Thorac Surg 2007;84:191-5. [Crossref] [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. A successful case of robotic bronchoplastic lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2013;19:478-80. [Crossref] [PubMed]
- Pan X, Chen Y, Shi J, et al. Robotic Assisted Extended Sleeve Lobectomy After Neoadjuvant Chemotherapy. Ann Thorac Surg 2015;100:e129-31. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [Crossref] [PubMed]
- Ishikawa N, Sun YS, Nifong LW, et al. Thoracoscopic robot-assisted bronchoplasty. Surg Endosc 2006;20:1782-3. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Berthet JP, Paradela M, Jimenez MJ, et al. Extended sleeve lobectomy: one more step toward avoiding pneumonectomy in centrally located lung cancer. Ann Thorac Surg 2013;96:1988-97. [Crossref] [PubMed]
- Zhou S, Pei G, Han Y, et al. Sleeve lobectomy by video-assisted thoracic surgery versus thoracotomy for non-small cell lung cancer. J Cardiothorac Surg 2015;10:116. [Crossref] [PubMed]
- He MC. Professor Jang-Ming Lee: how far will the robotic-assisted surgery go? J Thorac Dis 2015;7:780-2. [PubMed]
- Yang SM, Kuo SW, Lee JM. Robot-assisted thoracoscopic bronchoplasty. J Vis Surg 2015;1:20.


