Implications of the pulmonary artery to ascending aortic ratio in patients with relatively mild chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality and represents an important, preventable public health challenge (1). It is well known that forced expiratory volume in 1 sec (FEV1) and exercise capacity are associated with disease progression (2,3). However identifying specific biomarkers to predict progression and prognosis of mild COPD remains an important task.
Pulmonary hypertension is also associated with increased exacerbation risk and mortality in patients with COPD and is a particularly important predictor of morbidity and mortality in patients with severe COPD (4-6). However, pulmonary hypertension is not often used as a factor in clinical practice due to limitations in evaluating pulmonary hypertension and lack of available treatment. Right heart catheterization is the gold standard for diagnosing pulmonary hypertension, yet its use is limited by its invasive nature. Although transthoracic echocardiography is a readily available method, it is not sensitive for detecting pulmonary vascular disease in patients with COPD (7).
Recent advances in computed tomography (CT) technology make it useful for evaluating the intrinsic disease components of COPD, as well as the intrathoracic vasculature in these patients. The main pulmonary artery and the aorta are routinely described in patients undergoing chest CT. Relative pulmonary arterial enlargement, as determined by a pulmonary artery to ascending aortic ratio (PA-A ratio) >1, provides independent predictive information for exacerbation of COPD and has been suggested as a potential surrogate marker for pulmonary vascular disease (8,9), though these studies were evaluated in subjects with moderate to severe COPD .
However, little is known about the general implications of the PA-A ratio in patients with mild COPD. We evaluated the associations between clinical parameters and the PA-A ratio in a cohort with relatively mild COPD.
Methods
Subjects
Data for 131 patients diagnosed with mild to moderate COPD were analyzed retrospectively. Patients were selected from a Korean COPD cohort, which was developed to observe clinical outcomes of Koreans with COPD near cement plants. The study is recruiting subjects from 2012 until the end of 2015 (445 subjects had been recruited on 2015 July) and the subjects will be followed up for 10 years (10). Initial 142 patients with COPD having available clinical and CT data were selected among the 445 subjects, and 131 patients with mild to moderate COPD were included for analysis. Mild COPD was defined as post-bronchodilator FEV1/forced vital capacity (FVC) <0.7 and FEV1 >80% of the predicted value, and moderate COPD was FEV1/FVC <0.7 and FEV1 50% to 80%, as GOLD classification (2).
All patients were evaluated at the enrollment visit by medical interview, a physical examination, spirometry, laboratory tests, and a CT scan. Initial questionnaire data included demographics, disease history, residence location, environmental exposure, and patients reported exacerbations history. Exacerbations were defined as worsening symptoms (dyspnea, cough, or sputum) requiring treatment with systemic steroids or antibiotics, a visit to the emergency room, and/or admission to a hospital. Severe exacerbations were defined in case of admission to a hospital as worsening symptoms. The intensity and duration of respiratory symptoms, such as cough, sputum, dyspnea, and wheezing, were evaluated. Dyspnea was evaluated using the modified Medical Research Council Dyspnea grade. Health-related quality of life was evaluated by calculating the total score on the patient-reported COPD Assessment Test (CAT).
Spirometry was performed using an Easy One Kit (NDD, Zurich, Switzerland) before bronchodilation and 15 min after inhaling 400 µg salbutamol through a metered-dose inhaler with a spacer to assess increases in post-bronchodilator FEV1. Bronchodilator reversibility was evaluated by assessing the increase in post-bronchodilator FEV1 in liters. Airflow limitation was defined as a post-bronchodilator FEV1/FVC <0.7 (FEV1/FVC % <70). All pulmonary function tests were performed as recommended by the American Thoracic Society/European Respiratory Society (11).
Our Institutional Review Board approved the analyses of the clinical and imaging data (Institutional Review Board of Kangwon National University Hospital 2012, 06-007). Individual informed written consent was obtained from all patients.
Computed tomography (CT)
The volumetric CT scans were performed using a method reported previously (12). Volumetric CT scans were taken at full inspiration and expiration using a first-generation dual source CT system (Somatom Definition, Siemens Healthcare, Forchheim, Germany). Using in-house software, whole-lung images were extracted automatically, and the attenuation coefficient of each pixel was calculated. The emphysema index [volume fraction of the lung ≤950 Hounsfield units (HU)] and airway thickening (wall area percentage of two segmental bronchi: RB1 and LB1 +2) were quantified.
Based on a previous method, vascular measurements were taken on CT scans by an investigator who was unaware of the participant’s clinical characteristics (8). The PA-A ratio measurements were made from axial CT images on inspiration using digital imaging and communications in medicine (DICOM) software (OsiriX DICOM Viewer, ver. 4.0). A radiologist analyzed the CT images and used the same image to measure the diameters of the main pulmonary artery at the level of its bifurcation and the ascending aorta. The interpreter was blinded to the clinical information. Follow-up CT scans were performed 1 year later in a subset of the cohort.
Statistical analysis
Univariate and multivariate linear regression analyses were used to investigate factors associated with the PA-A ratio. The selected independent variables were age, sex, body mass index, total CAT score, pack-years of cigarette smoking, pre- and post-bronchodilator FEV1 (% of predicted), 1-year change in FEV1 (liter), history of exacerbations in previous year, and quantitative CT measurements (emphysema index and airway thickening, V950 and WA%). The significant variables in the univariate analysis were used in multivariate analysis. The SAS ver. 9.2 (SAS Institute, Cary, NC, USA) and SPSS ver. 15 (SPSS Inc., Chicago, IL, USA) statistical packages were used for the data analysis. A P value <0.05 was considered significant.
Results
Mean patient age was 73.3 (standard deviation, 6.6) years. Smoking history averaged 25.6 (18.3) pack-years. Post-bronchodilator FEV1 averaged 83% (17.8%) of predicted value. Men comprised 73.3% of the patients. The mean PA-A ratio was 0.78 (0.11) (Table 1). The histogram of PA-A ratio was showed on Figure 1. Among the 131 patients, 69 patients had mild COPD and 62 patients had moderate COPD.
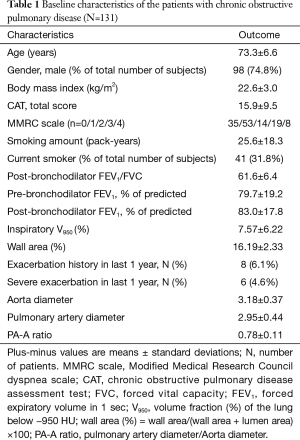
Full table
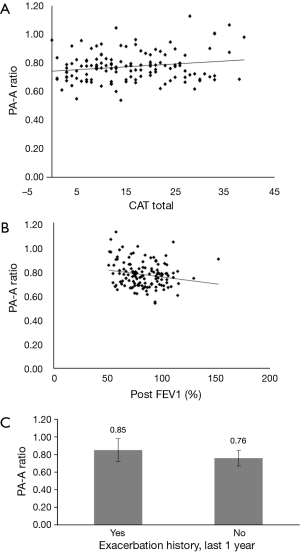
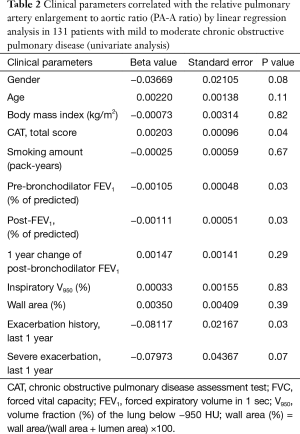
The univariate linear regression analysis revealed that CAT, pre- and post-bronchodilator FEV1, and history of exacerbations in the last year were associated with the PA-A ratio (Figure 2, Table 2). The multivariate linear regression analysis revealed that post-bronchodilator FEV1 (% of predicted) were independently correlated with the PA-A ratio (P=0.01) (Table 3).
Full table
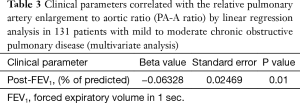
Full table
In 69 patients with mild COPD, total CAT score were significantly lower in patients group with decreased PA-A based on Korean reference values (0.79 in men and 0.85 in women) (Table 4) (13). The univariate and multivariate linear regression analysis revealed that total CAT score were also associated with the PA-A ratio in 69 patients with mild COPD (P=0.02) (Tables 5,6).
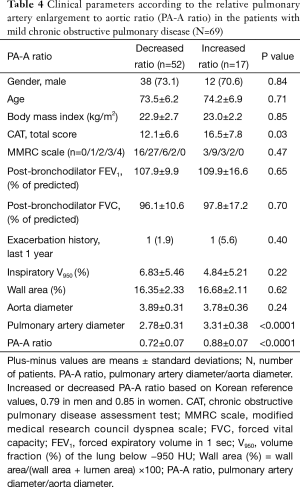
Full table
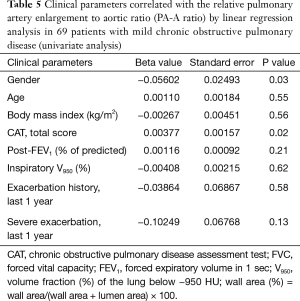
Full table
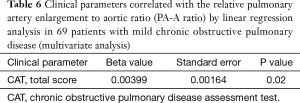
Full table
The PA-A ratio was measured in 69 patients during 1 year, and the mean change in the PA-A ratio was 0.01±0.06. The ratios of 30 subjects decreased, and the ratios of 39 subjects increased. The initial PA-A ratio and changes in the PA-A ratio during the year were not associated with changes in the clinical parameters during the year (Table 7).
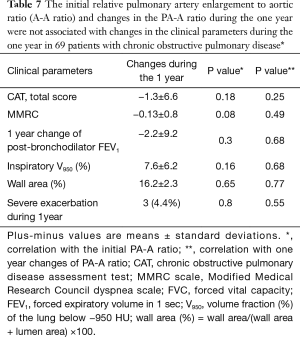
Full table
Discussion
Our results demonstrate that the PA-A ratio evaluated by CT imaging was independently associated with a representative pulmonary function parameter (FEV1, % of predicted value) in patients with mild to moderate COPD and independently associated with quality of life (total CAT score) in patients with mild COPD. These results suggest that the PA-A ratio, even ratios <1, may be an important biomarker for clinical outcome in patients with mild COPD.
Prior studies have shown that patients with COPD and pulmonary hypertension exhibit a linear decline in the 6-min walk test, a history of exacerbations, and a high mortality rate (14-16). Pulmonary hypertension in patients with COPD mainly occurs due to the hypoxic vasoconstriction that occurs during advanced airflow limitation (17,18) but could also occur due to other comorbid conditions in patients with milder disease. Nevertheless, pulmonary vascular changes may be an independent marker predicting a different disease history and a worse prognosis. Readily available and reproducible parameters of pulmonary vascular change may be a very important tool, such as FEV1, when managing patients with COPD. Right heart catheterization and transthoracic echocardiography are less useful in evaluating pulmonary vascular changes in COPD patients because of their invasiveness or insensitivity.
CT is an important tool to evaluate different characteristics and clinical outcomes in patients with COPD (19,20). Relative pulmonary arterial enlargement, as measured by CT (PA-A ratio), provides independent predictive information about patient outcome. A baseline PA-A ratio >1 is associated with severe exacerbations of COPD (8) and is an independent predictor of intermediate-term transplant-free survival (9). The PA-A ratio performs better than echocardiography for identifying pulmonary hypertension in patients with severe COPD (21). Moreover, the calculation metric of the PA-A ratio has good inter-observer and intra-observer agreement (22). Additionally, a recent report showed a three-dimensional (3D) approach to quantify the pulmonary artery volume predicted COPD exacerbations in ex-smokers with modest airflow limitation (23). It suggests that 3D total pulmonary artery volume measurement may be more sensitive than the PA-A ratio, by advancing more easy methods and validating on boarder range of patients. These results suggest that a readily available CT measure could be a potential surrogate for pulmonary vascular disease in patients with COPD.
Although the PA-A ratio as determined by a CT scan is promising as a biomarker in patients with COPD, its application remains limited to patients with moderate to severe disease (FEV1 <80% of the predicted value) and PA-A ratio values >1. A PA-A ratio >1 may only serve as a composite endpoint for advanced COPD disease or various other comorbid conditions. Mean FEV1 values in patient groups that show the predictive value of the PA-A ratio have been reported as 46 (% of predicted value), 37, and 29 (8,9,21). The reference PA-A ratio value in a large US reference sample was 0.77±0.09, and the 90th percentile ranged from 0.82–0.94, similar to that of patients with COPD (24). In addition, a Korean report showed that the reference PA-A ratio value in Korean populations was 0.79 in men and 0.85 in women (13). These results suggest that a lower PA-A ratio may indicate a milder COPD condition.
In our study, we demonstrated that the PA-A ratio was significantly associated with FEV1, as a representative pulmonary function parameter, in patients with relatively mild COPD (mean FEV1, 83% of predicted value). This result suggests that the PA-A ratio may be a marker for different disease courses and long-term outcome. We also determined that a PA-A ratio <1 may be an important quantitative marker for clinical outcome in patients with relatively mild COPD. In addition, we showed that the PA-A ratio was significantly associated with quality of life in patients with mild COPD (FEV1 >80% of predicted value), suggesting the PA-A ratio may be clinical marker even in patients with mild COPD. Thus, PA-A measurements from a CT scan raise hope for deciding early intervention in patients with mild COPD and for screening patients with severe COPD being considered for pulmonary hypertension therapy.
Our study had some limitations. First, the retrospective nature of this study and the small number of patients may prevent use of the PA-A ratio as a biomarker. Further prospective studies with a larger number of patients will be required to determine the predictive and prognostic values of the PA-A ratio. Second, we had a short-term follow-up of 1 year, which did not reveal meaningful changes in the PA-A ratio or clinical outcomes. Thus, a long-term follow-up study will identify more distinct implications of the PA-A ratio. Third, we had no cases of right heart catheterization, which is the gold standard for diagnosing pulmonary hypertension. If the PA-A ratio proves to be a useful independent predictive factor for clinical outcome in patients with COPD, we will have an important clinical parameter to gauge COPD.
In conclusion, the PA-A ratio evaluated by CT imaging was independently associated with FEV1 in patients with early mild to moderate COPD and independently associated with quality of life in patients with mild COPD. More research will be required to determine whether the PA-A ratio can be used as a biomarker for COPD progression or exacerbation events, independent of any information it provides about the pulmonary vasculature.
Acknowledgements
This study was supported by a grant from the Ministry of Environment, Republic of Korea.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board of Kangwon National University Hospital (No. 2012, 06-007) and written informed consent was obtained from all patients.
References
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765-73. [Crossref] [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-12. [Crossref] [PubMed]
- Kessler R, Faller M, Weitzenblum E, et al. "Natural history" of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med 2001;164:219-24. [Crossref] [PubMed]
- McGhan R, Radcliff T, Fish R, et al. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 2007;132:1748-55. [Crossref] [PubMed]
- Terzano C, Conti V, Di Stefano F, et al. Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung 2010;188:321-9. [Crossref] [PubMed]
- Laaban JP, Diebold B, Zelinski R, et al. Noninvasive estimation of systolic pulmonary artery pressure using Doppler echocardiography in patients with chronic obstructive pulmonary disease. Chest 1989;96:1258-62. [Crossref] [PubMed]
- Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012;367:913-21. [Crossref] [PubMed]
- Shin S, King CS, Brown AW, et al. Pulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstructive pulmonary disease. Respir Med 2014;108:1626-32. [Crossref] [PubMed]
- Hong Y, Kwon JW, Lee SA, et al. Methodology of an Observational Cohort Study for Subjects with Chronic Obstructive Pulmonary Disease in Dusty Areas Near Cement Plants. J Pulm Respir Med 2014;4:2.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Lee YK, Oh YM, Lee JH, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung 2008;186:157-65. [Crossref] [PubMed]
- Lee SH, Kim YJ, Lee HJ, et al. Comparison of CT-Determined Pulmonary Artery Diameter, Aortic Diameter, and Their Ratio in Healthy and Diverse Clinical Conditions. PLoS One 2015;10:e0126646. [Crossref] [PubMed]
- Sims MW, Margolis DJ, Localio AR, et al. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest 2009;136:412-9. [Crossref] [PubMed]
- Weitzenblum E, Hirth C, Ducolone A, et al. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax 1981;36:752-8. [Crossref] [PubMed]
- Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 2013;41:1292-301. [Crossref] [PubMed]
- Schulman LL, Lennon PF, Wood JA, et al. Pulmonary vascular resistance in emphysema. Chest 1994;105:798-805. [Crossref] [PubMed]
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on expert consensus documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573-619. [Crossref] [PubMed]
- Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006;173:1326-34. [Crossref] [PubMed]
- Lee JS, Lee SM, Seo JB, et al. Clinical utility of computed tomographic lung volumes in patients with chronic obstructive pulmonary disease. Respiration 2014;87:196-203. [Crossref] [PubMed]
- Iyer AS, Wells JM, Vishin S, et al. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest 2014;145:824-32. [Crossref] [PubMed]
- Wells JM, Dransfield MT. Pathophysiology and clinical implications of pulmonary arterial enlargement in COPD. Int J Chron Obstruct Pulmon Dis 2013;8:509-21. [Crossref] [PubMed]
- Lindenmaier TJ, Kirby M, Paulin G, et al. Pulmonary Artery Abnormalities in Ex-smokers with and without Airflow Obstruction. COPD 2016;13:224-34. [PubMed]
- Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography the Framingham heart study. Circ Cardiovasc Imaging 2012;5:147-54. [Crossref] [PubMed]


