Outcome of veno-venous extracorporeal membrane oxygenation use in acute respiratory distress syndrome after cardiac surgery with cardiopulmonary bypass
Introduction
Acute respiratory distress syndrome (ARDS) is a life-threatening pulmonary condition that can lead to rapidly progressive respiratory failure (1). Cardiac surgery under cardiopulmonary bypass (CPB) is a well-recognized risk factor of ARDS (2,3). CPB is known to trigger severe systemic inflammatory response, and abnormal cytokine release is known to be related to the interaction of blood components with the artificial surface of the CPB, surgical trauma, abnormal shear stress, and ischemia-reperfusion injury upon aortic declamping (4-6). Moreover, allogeneic blood product transfusions in patients undergoing cardiac surgery render them prone to transfusion-related acute lung injury (7). Other causes of postcardiotomy respiratory failure include ventilator-associated pneumonia, temporary cardiac dysfunction, systemic hypothermia, catecholamine infusions, and ventilator-induced lung injury (3,8).
Managements including lung protective ventilation strategy, strict fluid balance, and early use of neuromuscular blockade have demonstrated their efficacy (3). However, in the face of a rapid ARDS progression to a life-threatening hypoxemia, which is usually the case in post-cardiac surgical setting, prompt application of rescue therapies are often indicated to sustain life. For refractory severe ARDS, recruitment maneuvers, nitric oxide inhalation, or prone positioning may transiently improve oxygenation. However, evidence of safety and effectiveness of these treatments in patients of recent open-heart surgery is limited, and survival benefits have rarely been reported.
Over the past 2 decades, veno-venous extracorporeal membrane oxygenation (VV-ECMO) support has emerged as a valuable rescue therapy to facilitate respiratory recovery in refractory ARDS to conventional therapy (9). With technical improvements in modern ECMO (10-12) and several recent clinical data reporting encouraging survival after VV-ECMO for adult patients with respiratory failure (13,14), the application of VV-ECMO is sharply increasing in broader clinical conditions (12,15,16). Although the identification of patients at high risk for postcardiotomy ARDS remains an area of active investigation (2,8,17,18), the standard management is not decided yet. The use of VV-ECMO for respiratory failure after thoracic surgery has increased and is known to improve survival in patients with ARDS perioperatively. Veno-arterial ECMO (VA-ECMO) therapy could be valuable for patients with postcardiotomy cardiac failure. We aimed to analyze the treatment outcome in patients who required VV-ECMO support for postcardiotomy ARDS despite other rescue modalities.
Methods
Patients
A total of 2,234 patients underwent cardiac surgery at Severance Cardiovascular Hospital, Yonsei University College of Medicine, between March 2013 and February 2016. From a retrospective review of the ECMO database in our intensive care unit (ICU), we identified 13 adult patients who received VV-ECMO for development of refractory ARDS after cardiac surgery under CPB. We excluded patients who died on the day of surgery. Preoperative and postoperative data as well as laboratory and respiratory data at the initiation and during ECMO therapy were obtained using a retrospective review of the prospectively recorded registry database and electronic medical records. This study was approved by the institutional review board of Yonsei University Health Service, Severance Hospital, and was conducted in accordance with the Declaration of Helsinki.
Indication of veno-venous extracorporeal membrane oxygenation (VV-ECMO)
All study patients were transferred to the cardiovascular surgical ICU with endotracheal intubation under mechanical ventilation support after surgery. Arterial blood gas analysis was routinely examined perioperatively and immediately upon ICU admission. Blood pressure and sugar level were strictly monitored. In patients developing postoperative respiratory failure, an attempt to improve oxygenation with conventional ventilation is generally undertaken including optimizing positive end-expiratory pressure (PEEP) level or prone positioning. Nitric oxide was not used per institutional policy. If stabilization efforts were not successful after several hours and the patient had severe hypoxemia with a partial pressure of arterial oxygen-to-fraction of inspired oxygen ratio (PaO2/FiO2) ≤85 mmHg and PEEP ≥5 cmH2O or if uncompensated respiratory acidosis with pH ≤7.15 combined with new bilateral pulmonary infiltrates on chest radiograph persisted, VV-ECMO was considered. All but 1 patient (patient 3) met the criteria for severe ARDS based on the Berlin definition (19) at the time of VV-ECMO implantation. Patient 3 who had underlying chronic obstructive pulmonary disease required VV-ECMO for acute exacerbation of chronic obstructive pulmonary disease with uncontrolled respiratory acidosis (pH 7.15).
Implantation and management of veno-venous extracorporeal membrane oxygenation (VV-ECMO)
All VV-ECMOs were performed with percutaneous cannulation by trained cardiovascular surgeons. The femoral vein was directly cannulated after limited cut-down using the Seldinger technique to introduce a 21 F venous cannula (DLP Medtronic, Minneapolis, MN, USA), with the tip positioned in the inferior vena cava at the entrance of the right atrium for drainage. The oxygenated blood was returned through a 17 F arterial cannula (DLP Medtronic) in the right internal jugular vein with the tip positioned in the superior vena cava just before the entrance to the right atrium. The rest of the ECMO circuit consisted of a centrifugal pump oxygenator with hollow-fiber polymethylpentene membrane heparin-bound surface using a PLS bypass system (MAQUET; Cardiopulmonary AG, Hechingen, Germany; n=9) or with Xcoating Capiox emergent bypass system (Terumo, Tokyo, Japan; n=4).
The sweep gas flow was set to maintain a PaCO2 target of 35−45 mmHg under lung rest ventilation strategy. The target ECMO blood pump speed was 3.0−4.5 L/min. Systemic anticoagulation with unfractionated heparin was titrated to maintain an activated clotting time between 160 and 220 s, but heparin was replaced by argatroban in 1 patient (patient 10) who developed heparin-induced thrombocytopenia. Supplement of coagulation factors or platelets were applied as indicated by laboratory coagulation analysis. Under VV-ECMO, the general targets of lung-protective ventilation were tidal volumes of 3−5 mL/kg predicted body weight, respiratory rate of 12−16/min, and a plateau inspiratory pressure <25 cmH2O with low FiO2 (≤60%). The PEEP was set at 6−10 cmH2O, but it could be changed at the discretion of the physician according to patients’ status.
Veno-venous extracorporeal membrane oxygenation (VV-ECMO) weaning
In patients with improvement of lung functions evident in hemodynamic, clinical, and respiratory measurements, ECMO flow was gradually reduced by approximately 0.5 L/min at a time. When adequate oxygenation was sustained with moderate ventilator settings (FiO2 <0.6, PEEP <10 mmHg, peak inspiratory pressure <25 cmH2O, and respiratory rate <40/min) for at least 4 h, VV-ECMO was stopped. VV-ECMO weaning was determined successful if patients survived at least 72 h after ECMO removal.
Statistical analysis
Continuous variables were analyzed by Mann-Whitney U test and were demonstrated as mean ± standard error or median with range. Categorical data were analyzed by Fisher’s exact test, and these data were shown as absolute frequencies and percentages. The overall survival curve after implementation of ECMO was plotted using the Kaplan-Meier method, and differences were evaluated using the log-rank test. Two-sided P values <0.05 were considered statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences version 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient baseline characteristics
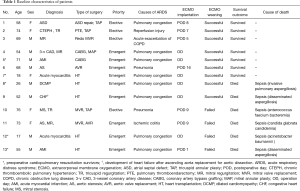
During the study period, 13 adult patients (mean age, 54.7±5.9 years) underwent VV-ECMO for ARDS after cardiac surgery under CPB. The characteristics of the study population are listed in Table 1. Patient 9 had undergone previous ascending aorta replacement for aortic dissection, and patient 3 had undergone a redo mitral valve replacement. The surgeries included coronary artery bypass graft (CABG; n=1), CABG with valvular operation (n=1), valvular operation (n=6), and emergent heart transplantation (HT; n=5). Four of the 8 non-HT surgeries were emergent operations for 3-vessel coronary artery disease (n=2) or severe aortic stenosis (n=2). Indications for HT were acute myocarditis (n=2), dilated cardiomyopathy with mitral regurgitation (n=1), congestive heart failure after ascending aorta replacement for aortic dissection (n=1), and acute myocardial infarction with cardiac arrest (n=1), as shown in Table 1. Preoperative VA-ECMO support as a bridge therapy was required in all 5 HT patients with a median preoperative interval of 6 days (range, 2−8 days). Four (80%) of these patients survived cardiac arrest after cardiopulmonary resuscitation. An underlying chronic obstructive pulmonary disease was noted in 2 patients.
Full table
Extracorporeal membrane oxygenation (ECMO) institution
The mean CPB duration (206±22 vs. 150±20 min; P=0.107) and aortic cross-clamping time (123±11 vs. 83±6 min; P=0.007) were longer in patients with HT (data not shown). All but 1 patient among the 8 non-HT patients were weaned from CPB without difficulty or inotropes. Patient 5 who had received an isolated CABG under CPB for acute myocardial infarction with cardiogenic shock required intra-aortic balloon pump administration during a re-exploration for pleural space bleeding, and the configuration of the preoperative VA-ECMO was switched to VV-ECMO due to severe hypoxemia. Patient 11 also underwent re-exploration for aortomy site bleeding on postoperative day 1, which was controlled successfully. The median interval to VV-ECMO from cardiac surgery among the 8 non-HT patients was 7.5 days (range, 1–16 days). All 5 HT patients initiated VV-ECMO before being transferred to the ICU.
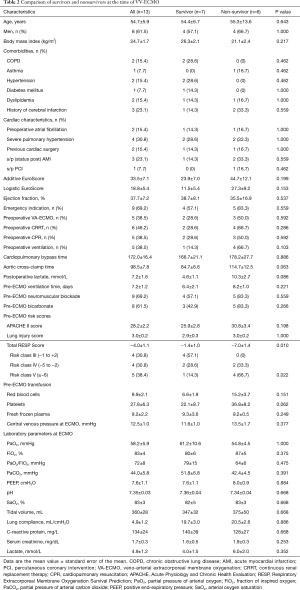
The clinical characteristics at the time of VV-ECMO initiation are described in Table 2. Six patients were receiving continuous veno-venous hemodiafiltration. The mean lung injury score and Acute Physiology and Chronic Health Evaluation (APACHE) II score at VV-ECMO implantation were high (3.0±0.2 and 28.2±2.2, respectively), reflecting severe disease. We also used the recently proposed prognostic scoring system, the Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score (20), in our study patients (Table 2).
Full table
Extracorporeal membrane oxygenation (ECMO) and survival outcomes
VV-ECMO weaning was successful in 9 (69%) of the 13 patients. The median duration of VV-ECMO in these patients were 7.2 days (range, 3−16 days). VV-ECMO reimplantation was not observed. The average amounts of transfusions were 12.6±2.8 units of red blood cell, 53.8±13.4 units of platelets, and 6.4±2.5 fresh frozen plasma. Non-survivors received significantly larger amounts of post-ECMO platelet transfusions (67.2±23.3 vs. 45.4±16.9; P=0.037). After a median follow-up duration of 14.5 months (range, 1.0−33.0 months) for survivors, the 1-year overall survival was 58.6%±14.4%. Overall, in-hospital mortality was 46.1%. Six patients, including 2 successfully weaned cases (patient 8 and patient 9), died during their ICU stay after a median of 5.5 days (range, 1−35 days) after ECMO implantation and a median of 26 days (range, 1−201 days) after cardiac surgery. Four were HT patients, and septic shock from fungal (n=4) or bacterial (n=2) bloodstream infections accounted for all cause of deaths in the present series. Patient 9 had achieved successful ECMO weaning but died of disseminated aspergillosis with concomitant critical neuromyopathy and consequent prolonged mechanical ventilation. Patient 11 had ischemic colitis on postoperative day 9, necessitating descending colectomy with colostomy, which was responsible for the fungemia (Table 1). Major ECMO-related complications such as leg ischemia were not observed in our patient cohort. All 7 (54%) hospital survivors were currently alive at median follow-up of 16 months (range, 7−34 months).
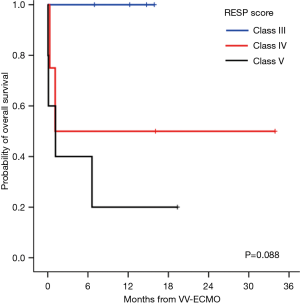
Survivors (n=7) and non-survivors (n=6) were comparable for age, sex, body mass index, comorbidities, and both additive and logistic EuroScores, as shown in Table 2. However, non-survivors required higher units of perioperative blood transfusions, had longer aortic cross-clamping time, and had higher APACHE II score and lactate level at ECMO initiation, but did not reach statistical significance. Interestingly, the only pre-ECMO prognostic factor for survival was the RESP score. The risk classes according to the total RESP score were class III (scores −1 to 2) in 4 patients, class IV (scores −5 to −2) in 4 patients, and class IV (scores ≤6) in 5 patients. All non-survivors were classified as risk class IV or V, and the mean RESP score was significantly lower (−7±1.4) compared to that of the survivors (−1.4±1.0; P=0.01). As shown in Figure 1, the differences in the Kaplan-Meier survival from VV-ECMO initiation after cardiac surgery according to the RESP risk classes were identified with a borderline significance (100% in class III, 50%±25% in class IV, and 20%±17.9% in class V; P=0.088).
Discussion
Despite the advances in CPB techniques and in the preventive measures of respiratory complications after cardiac surgery (8,21), postoperative ARDS manifests in 10−20% of patients who undergo cardiac surgery (2), and its overall mortality remains high (3,18,22). According to a recent literature review on ARDS after cardiac surgery (22), the mortality rate varied from 15% to 92%, suggesting a heterogeneity in the study populations, varied definitions used to identify ARDS, and different managements. Optimized mechanical ventilation in line with the ARDS network is a key priority for physicians in managing ARDS (23), and this lung-protective ventilation strategy of limiting tidal volume to 6−8 mL/kg predicated body weight while keeping the plateau airway pressure below 30 cmH2O is of particular importance for patients receiving cardiac surgery under CPB, in whom excessive systemic inflammation has already been induced by intraoperative extracorporeal circulation (3,24,25). According to a recent study on 3,434 patients receiving cardiac surgery by Lellouche et al. (26), the use of tidal volumes >12 mL/kg predicted body weight during the postoperative period was associated with prolonged mechanical ventilation and increased risk of organ failure.
Despite these backgrounds, in some patients with severe ARDS, adequate oxygenation can only be achieved using aggressive, non-protective ventilation. To obviate ventilation-induced lung injury while ensuring adequate oxygen delivery to vital organs, timely application of ECMO should be considered. Whereas the use of VA-ECMO for refractory cardiovascular dysfunction after cardiac surgery has been well described in literature demonstrating its efficacy as a bridge to recovery (27-29), there is paucity of data on the use of postcardiotomy VV-ECMO for postoperative life-threatening respiratory dysfunction. VA-ECMO should be avoided in patients with respiratory failure without cardiac failure or refractory shock due to many potential complications, including systemic thromboembolism, differential hypoxia from maldistribution of oxygen, and left ventricular distention leading to pulmonary edema and aggravation of pulmonary injury (12,30).
We report herein the largest series of adult patients who received VV-ECMO after cardiac surgery under CPB in the context of postoperative refractory ARDS. We documented that VV-ECMO was a feasible rescue therapy for adult patients with life-threatening ARDS after cardiac surgery under CPB. Although significant morbidity associated with VV-ECMO was not observed in the present case series, successful weaning from VV-ECMO was possible in 69% of the 13 study cases. Not surprisingly, survival outcome was less satisfactory among the HT cases (n=5) in whom the hospital discharge rate was only 20%, whereas 75% of the non-HT cases (n=8) achieved a long-term survival outcome.
Recently, several investigator groups have developed prognostic models aimed to facilitate the identification of high-risk populations for ARDS. Gajic et al. (2) developed the LIPS model by including both medical and surgical patients with ARDS for analysis. Kor et al. (31) proposed the surgical lung injury prediction (SLIP) model for use in an elective surgical population. More recently, the SLIP-2 score, the refined SLIP model (18), was developed and suggested a better prognostic value in identifying patients at high risk of ARDS in a higher-risk surgical population. All these efforts will help minimize the incidence of postoperative ARDS and its severity. In our study, CPB time, a known variable prognostic of postcardiotomy ARDS and survival (3,21,22,24,25), was not significantly different between survivors and non-survivors, whereas the aortic cross-clamping time tended to be longer in the non-survivors, as suggested by others (32).
Meanwhile, prognostic factors for post-VV-ECMO survival outcomes in refractory ARDS after cardiac surgery have not been yet reported. Recently, Schmidt et al. (20) published a novel mortality prediction model called the RESP score, developed from ECMO data of >2,000 patients from the ELSO registry. Klinzing et al. (33) recently reported a better performance of this score compared to the Predicting Death for Severe Acute Respiratory Distress Syndrome on Veno-venous Extracorporeal Membrane Oxygenation score in their external validation on 51 patients receiving VA-ECMO or VV-ECMO support. Even in our present small series, the RESP score was significantly associated with survival outcomes. Although all 13 cases were classified as classes III–V, large differences in overall survival according to the class were observed, although only borderline significance was demonstrated.
The major limitation of our study is the retrospective study design and the challenge of meaningful statistical significance. Because of the small number of patients, no final conclusion can be drawn from the performance of the RESP score in our study. The decision for timely application of ECMO support in the context of postcardiotomy respiratory failure remains difficult and should be determined based on individual considerations because ECMO support is associated with high morbidity. In this context, our data may suggest that the RESP score can be useful in the prediction of post-ECMO survival outcomes in a diverse patient population.
In conclusion, VV-ECMO seems a feasible bridging therapy for injured lung to recover in severe refractory ARDS after cardiac surgery under CPB. As all deaths occurred after septic shock from bloodstream infection acquired during ECMO, knowledge of high-risk patients (i.e., HT recipients) and common causal organisms may enhance strategies to prevent and control infectious consequence and increase post-ECMO survival. Although the prognostic value of the RESP score risk classification validated in this study should be interpreted with caution, given the relatively small number of patients, the RESP score seems a valuable tool for physicians to carefully balance the high perioperative morbidity and resource use in ECMO treatment against the probability of survival.
Acknowledgements
We acknowledge all members at the intensive care units for their excellent care of the patients.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of Yonsei University Health Service, Severance Hospital, and was conducted in accordance with the Declaration of Helsinki.
References
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [Crossref] [PubMed]
- Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 2011;183:462-70. [Crossref] [PubMed]
- Stephens RS, Shah AS, Whitman GJ. Lung injury and acute respiratory distress syndrome after cardiac surgery. Ann Thorac Surg 2013;95:1122-9. [Crossref] [PubMed]
- Rothenburger M, Soeparwata R, Deng MC, et al. The impact of anti-endotoxin core antibodies on endotoxin and cytokine release and ventilation time after cardiac surgery. J Am Coll Cardiol 2001;38:124-30. [Crossref] [PubMed]
- Reis Miranda D, Gommers D, Struijs A, et al. Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. Eur J Cardiothorac Surg 2005;28:889-95. [Crossref] [PubMed]
- Milot J, Perron J, Lacasse Y, et al. Incidence and predictors of ARDS after cardiac surgery. Chest 2001;119:884-8. [Crossref] [PubMed]
- Vlaar AP, Hofstra JJ, Determann RM, et al. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: a prospective nested case-control study. Blood 2011;117:4218-25. [Crossref] [PubMed]
- Apostolakis EE, Koletsis EN, Baikoussis NG, et al. Strategies to prevent intraoperative lung injury during cardiopulmonary bypass. J Cardiothorac Surg 2010;5:1. [Crossref] [PubMed]
- Schmidt M, Hodgson C, Combes A. Extracorporeal gas exchange for acute respiratory failure in adult patients: a systematic review. Crit Care 2015;19:99. [Crossref] [PubMed]
- Turner DA, Cheifetz IM. Extracorporeal membrane oxygenation for adult respiratory failure. Respir Care 2013;58:1038-52. [Crossref] [PubMed]
- Müller T, Philipp A, Luchner A, et al. A new miniaturized system for extracorporeal membrane oxygenation in adult respiratory failure. Crit Care 2009;13:R205. [Crossref] [PubMed]
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012;38:210-20. [Crossref] [PubMed]
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, et al. Extracorporeal membrane oxygenation for 2009 Influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009;302:1888-95. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- McCarthy FH, McDermott KM, Kini V, et al. Trends in U.S. extracorporeal membrane oxygenation use and outcomes: 2002-2012. Semin Thorac Cardiovasc Surg 2015;27:81-8. [Crossref] [PubMed]
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Chen SW, Chang CH, Chu PH, et al. Risk factor analysis of postoperative acute respiratory distress syndrome in valvular heart surgery. J Crit Care 2016;31:139-43. [Crossref] [PubMed]
- Kor DJ, Lingineni RK, Gajic O, et al. Predicting risk of postoperative lung injury in high-risk surgical patients: a multicenter cohort study. Anesthesiology 2014;120:1168-81. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014;189:1374-82. [Crossref] [PubMed]
- García-Delgado M, Navarrete-Sánchez I, Colmenero M. Preventing and managing perioperative pulmonary complications following cardiac surgery. Curr Opin Anaesthesiol 2014;27:146-52. [Crossref] [PubMed]
- Weissman C. Pulmonary complications after cardiac surgery. Semin Cardiothorac Vasc Anesth 2004;8:185-211. [Crossref] [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Hirai S. Systemic inflammatory response syndrome after cardiac surgery under cardiopulmonary bypass. Ann Thorac Cardiovasc Surg 2003;9:365-70. [PubMed]
- Apostolakis E, Filos KS, Koletsis E, et al. Lung dysfunction following cardiopulmonary bypass. J Card Surg 2010;25:47-55. [Crossref] [PubMed]
- Lellouche F, Dionne S, Simard S, et al. High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology 2012;116:1072-82. [Crossref] [PubMed]
- Rastan AJ, Dege A, Mohr M, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010;139:302-11, 311.e1.
- Hsu PS, Chen JL, Hong GJ, et al. Extracorporeal membrane oxygenation for refractory cardiogenic shock after cardiac surgery: predictors of early mortality and outcome from 51 adult patients. Eur J Cardiothorac Surg 2010;37:328-33. [PubMed]
- Doll N, Kiaii B, Borger M, et al. Five-year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg 2004;77:151-7; discussion 157. [Crossref] [PubMed]
- Cove ME. Disrupting differential hypoxia in peripheral veno-arterial extracorporeal membrane oxygenation. Crit Care 2015;19:280. [Crossref]
- Kor DJ, Warner DO, Alsara A, et al. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology 2011;115:117-28. [Crossref] [PubMed]
- Slottosch I, Liakopoulos O, Kuhn E, et al. Outcomes after peripheral extracorporeal membrane oxygenation therapy for postcardiotomy cardiogenic shock: a single-center experience. J Surg Res 2013;181:e47-55. [Crossref] [PubMed]
- Klinzing S, Wenger U, Steiger P, et al. External validation of scores proposed for estimation of survival probability of patients with severe adult respiratory distress syndrome undergoing extracorporeal membrane oxygenation therapy: a retrospective study. Crit Care 2015;19:142. [Crossref] [PubMed]


