Liver failure in total artificial heart therapy
Introduction
Congestive hepatopathy (CH) and acute liver failure (ALF) are common among biventricular heart failure patients (1). In chronic cases, high right atrial pressure causes venous congestion, sinusoidal stasis, parenchymal atrophy, necrosis and ultimately hepatic fibrosis. In acute cases and especially in cardiogenic shock, hypoperfusion and ischemia result in hepatic necrosis. The positive impact of total artificial heart (TAH) on end-organ function is well known (2). With this study we sought to evaluate the extent of hepatic recovery in CH and ALF cases and the associated clinical outcome.
Methods
Between July 2011 and February 2015, 31, consecutive, male, Caucasian patients received a SynCardia-TAH (SynCardia Systems Inc., Tucson, USA). Preoperative transaminase levels, such as serum glutamic oxaloacetic transaminase (sGOT) and serum glutamic-pyruvic transaminase (sGPT), bilirubin and International Normalized Ratio (INR) taken 24 hours prior to surgery, as well as clinical characteristics were used for assigning the patients in three groups. Model of End-Stage Liver Disease (MELD) and Model of End-Stage Liver Disease Excluding INR (MELD-XI) scores were unfortunately not universally applicable, as many patients were already intubated and sedated and therefore could not be safely evaluated for the presence of hepatic encephalopathy. Institutional Review Board approval was not necessary. Informed patient consent for data collection and analysis was obtained prior to the TAH procedure.
The first group was comprised of 17 patients with normal liver function (sGOT and sGPT under 50 U/L, total bilirubin under 1.1 mg/dL with isolated abnormalities of each parameter allowed, but not higher than 80% of these values). Median patient age was 64 years (range, 44–81 years). The most common diagnosis was ischaemic cardiomyopathy, numbering nine patients. Six patients had an Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) 1 profile and 11 patients had an INTERMACS 2 profile. Thirteen patients were treated with the intention of Bridge-to-Transplant.
The second group consisted of five patients exhibiting ALF (at least two of the following: GOT >500 U/L, GPT >400 U/L, bilirubin >2 mg/dL, ammonia>80 μg/dL, intracranial hypertension when measured, INR >1.7 on the absence of anticoagulants that developed in less than 24 hours). Median age was 66 years (range, 49–74 years). Two patients were diagnosed with ischaemic cardiomyopathy (P=0.31). Four patients were at INTERMACS level 1 (P=0.04). Two patients were treated with the intention of Bridge-to-Transplant (P=0.06).
The third group was comprised from 9 patients (sGOT <150 U/L, sGPT <100 U/L, bilirubin >1.7 mg/dL or INR >1.7 on the absence of anticoagulants). Median age was 63 years (range, 50–77 years). The most common diagnosis was ICM, which accounted for seven patients (P=0.11). Four patients were ranked as INTERMACS 1 (P=0.06). Six patients were treated with the intention of Bridge-to-Transplant (P=0.30).
Bilirubin, sGOT and sGPT levels were monitored routinely as part of the standard biochemical blood controls. Preoperative values (24 hours before surgery), postoperative values (on the day following the surgery) and values after 1, 2, 4, 6 weeks were chosen to evaluate the course of liver function. Furthermore, liver failure-associated mortality for each group was calculated. We compared the liver failure associated-mortality results of the second and third with the first group using a two sample t-test. Chi-square test was used to determine influence of preoperative bilirubin levels on postoperative survival. As liver failure-associated mortality we defined mortality due to fulminant hepatic failure with extreme derangements of transaminases and bilirubin, severe coagulopathy in the absence of anticoagulants, ammonia increase, persistent hypoglycaemia, and presence of hepatorenal syndrome resulting in haemodynamic instability and death, when other possible death causes such as cardiac tamponade, hemorrhage or sepsis can be definitely excluded.
Results
Patient data were prospectively collected and retrospectively analyzed.
In the group with preoperative normal liver function only one of the seventeen patients presented a postoperative ALF with elevation of transaminases, massive accumulation of bilirubin and prolongation of INR with excessive bleeding needing substitution with prothrombin complex concentrate, FFPs (Fresh Frozen Plasma), Factor VIIa, fibrinogen and tranexamic acid. This event was triggered by a period of haemodynamic instability due to a massive retro- and intraperitoneal bleeding. The median sGOT and sGPT values of the group showed a declining trend. Bilirubin showed a fluctuation with a moderate rise, peaking after two weeks, and decreasing thereafter (see Figures 1-3). This was attributed to biliary sludge formation due to bowel rest and bile stasis during parenteral nutrition. Sludge formation was confirmed sonographically. Liver failure-associated mortality was 0%. Overall mortality was 58.82% (10/17). Mortality causes included massive stroke (4 patients), sepsis (3 patients), cerebral haemorrhage (1 patient), cardiac tamponade (1 patient) and massive pulmonary embolism (1 patient).
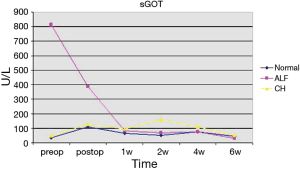
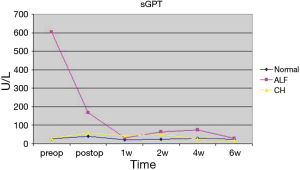
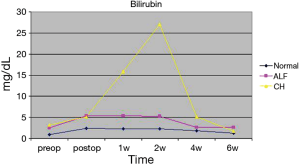
In the group of five patients demonstrating a preoperative ALF, all showed an immediate postoperative aminotransferase reduction. The reduction in median values was 52.5% (from 816 U/L, range 64–4,373 U/L, to 387 U/L, range 124–631 U/L) for sGOT and 72.2% (from 605 U/L, range 51–2,173 U/L, to 168 U/L, range 37–407 U/L) for GPT. Median bilirubin values increased (from 2.56 mg/dL, range 1.10–27.14 mg/dL, to 5.4 mg/dL, range 9.03–18.58 mg/dL) after the first 24 hours. Bilirubin remained elevated during the following 5 weeks and returned to preoperative levels after 6 weeks (median 2.63, range 0.85–54.17 mg/dL) (see Figures 1-3). One patient showed further deterioration of hepatic function and ultimately died. Overall and liver-associated mortality in this group was 20% (P=0.03).
The third group, comprised of patients with preoperative CH, showed mixed results. Five out of nine patients developed a deterioration of hepatic function with no or moderate rise in aminotransferase levels but with massive increase of bilirubin and derailment of coagulation. Preoperative median sGOT, sGPT and bilirubin values were 48 U/L (range, 25–128 U/L), 29 U/L (range, 4–75 U/L) and 3.21 mg/dL (range, 1,79–12,02 mg/dL). Median sGOT and sGPT values twenty-four hours after the surgery were 127.5 U/L (range, 37–210 U/L) and 59 U/L (range, 10–135 U/L) respectively. Bilirubin increased 24 hours after surgery with a median value of 5.17 mg/dL (range, 3.43–16.35 mg/dL), reaching a peak in two weeks (median 27.02 mg/dL, range 0.75–62.32 mg/dL) (Figures 1-3). Liver-associated mortality was 44.44% (P=0.0008). Overall mortality was 66.6% (6/9). Apart from liver failure associated deaths (4 patients), other mortality causes include bowel perforation (1 patient) and sepsis (1 patient).
Discussion
The biochemical findings could be explained by the anatomy of the liver. Its dual blood supply makes it resistant to haemodynamic-induced hepatocyte necrosis with the exception of severe or prolonged periods of hypoperfusion. On the other hand, elevated pressure in the hepatic veins, leading to sinusoidal stasis, contributes to impaired hepatocyte function and produce cholestatic effects (3). It is also known that total bilirubin is correlated with the elevation in central venous pressure, and therefore hepatic congestion, in patients with right ventricular failure or tricuspid insufficiency (2). Moreover, elevated total bilirubin is a risk factor for patients undergoing Left Ventricular Assist Devices (LVAD) implantation (4), as it reflects the degree of right ventricular impairment prior to surgery. Applying the above-mentioned to the group of patients with CH, we observe a significantly elevated preoperative bilirubin (median: 3.21 mg/dL, range: 1.79–12.02 mg/dL) in comparison to the patients having a normal liver function (median: 0.92 mg/dL, range: 0.24–1.91 mg/dL) (see Table 1). This finding indicates a sustained hepatic congestion leading to severe hepatocyte malfunction prior to surgery. Transaminases were normal or mildly elevated prior to surgery even in patients with a notable rise in bilirubin levels. Furthermore, patients, who suffered from a postoperative deterioration of hepatic function in this group, either maintained normal or showed slightly elevated transaminases despite increases in bilirubin exceeding 40 mg/dL and development of severe coagulopathy. As a conclusion, bilirubin constitutes an important marker of hepatocyte dysfunction regardless of transaminase levels as well as a risk factor in patients with CH. In this study we observed that patients with a bilirubin level >3 mg/dL have a higher perioperative mortality in comparison to patients with bilirubin levels <3 mg/dL (100% vs. 25%; Chi-square =5.6; P=0.018). However due to the small patient sample a general recommendation cannot be formulated. This still has to be the subject of larger, prospective studies.

Full table
The experience gained from the above-mentioned is that the effect of TAH-implantation on hepatic function varies according to the preoperative liver status of the patient. Patients without preexisting acute or chronic liver failure or CH are unlikely to develop them after surgery. Patients with preexisting CH due to chronic right ventricular dysfunction exhibit an increased overall and liver associated mortality, which can be connected to preoperative bilirubin levels.
Acknowledgements
None.
Footnote
Conflicts of Interest: G Tenderich is active as a surgical proctor by SynCardia Systems, Inc. None of the other authors is affiliated with SynCardia Systems, Inc. or any other manufacturers of commercial products discussed in the manuscript. Furthermore the authors disclose any discussion of unapproved use of any pharmaceutical or medical device in the manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: Institutional Review Board approval was not necessary. Informed patient consent for data collection and analysis was obtained prior to the TAH procedure.
References
- Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail 2009;11:170-7. [Crossref] [PubMed]
- Copeland JG, Smith RG, Arabia FA, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med 2004;351:859-67. [Crossref] [PubMed]
- Giallourakis CC, Rosenberg PM, Friedman LS. The liver in heart failure. Clin Liver Dis 2002;6:947-67. [Crossref] [PubMed]
- Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008;51:2163-72. [Crossref] [PubMed]


