Uniportal video-assisted thoracic surgery resection of small ground-glass opacities (GGOs) localized with CT-guided placement of microcoils and palpation
Introduction
Uniportal video-assisted thoracic surgery (VATS) is constantly evolving and becoming increasingly popular (1,2); however, wedge resection of small pulmonary ground-glass opacities (GGOs) (<15 mm) is very challenging, especially deeply situated subpleural GGOs, by means of uniportal VATS. The habitual practice of palpation is often inconclusive due to the lack of solid lung background. Furthermore, hookwire and methylene blue injection are not very helpful with lesions in the blind areas (3). CT-guided microcoil localization and fluoroscopically guided VATS resection cause radiation exposure for medical staff and patients during surgery. We describe our approach of utilizing radiologically guided microcoil localization with palpation in uniportal VATS wedge resection.
Operative techniques
This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital.
Patients
Thirteen patients (6 males and 7 females) with deeply situated subpleural GGOs underwent successful uniportal wedge resection after CT-guided microcoil localization from January to December 2014. The average age was 47.3±11.7 years (range, 25 to 64 years). The GGO size was 0.9±0.2 mm (range, 8 to 14 mm), and the distance to the closest visceral pleura was 20±5 mm (range, 5 to 30 mm). The selection criteria were based on the following CT findings: lesion diameter ≤15 mm, distance from pleural surface >5 mm, pGGO or a lesion mostly comprised of GGOs. Patients with a history of pleurisy and failure of microcoil localization were excluded.
Microcoil localization technique
The CT-guided percutaneous microcoil nodule localization procedure was performed using conscious sedation and local anesthesia. CT scan was used to plan the needle access route and measure the depth of the nodule from the external surface of the chest wall.
The core of a 150-mm-long Chiba biopsy needle was removed. A 30-mm-long, 0.035-inch-diameter fiber-coated stainless steal microcoil was pushed into the Chiba needle by using the stiff end of a 40-cm-long, 0.018-inch-diameter guidewire from a micropuncture catheter introducer set. After the microcoil was loaded into the needle, a hemostat was used to mark the pusher wire at one point—the length necessary to eject the entire 30-mm-long microcoil from the Chiba needle.
All microcoil localizations were performed or closely supervised by one interventional chest radiologist with 20 years’ experience. With CT guidance, the loaded Chiba needle was pushed through and 5 mm deep to the suspicious nodule. The premeasured guidewire was advanced into the Chiba needle up to the mark at which the hemostat was placed, and the 30-mm-long microcoil was deployed into the lung parenchyma, where it assumed a tightly coiled ball configuration just beyond the tip of the needle. A localizer CT scan was obtained to confirm the microcoil deployment. The empty needle and guidewire assembly were then withdrawn (Figure 1). During the localization, we preferred a relatively longer travel from entry point of visceral pleura to the lesion to prevent microcoil displacement to the cavity. To avoid dislocation, we push in the guiding wire when retrieving the Chiba needle to keep the microcoil relatively immobilized. When Chiba needle is retrieved and not in contact with the microcoil, we then pull out the guiding wire.
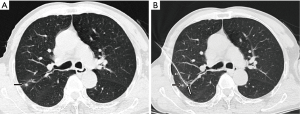
A postprocedural larger-volume CT scan was obtained to assess the final position of the microcoil relative to the nodule and the pleural space and to test for the presence of postprocedural hemorrhage, as indicated by new airspace consolidation and pneumothorax. The patient was then transferred to the preoperative area, where he or she waited for 1–4 hours before undergoing surgical resection of the nodule in the operating room. There were two pneumothoraxes and one intrapulmonary hemorrhage, post-procedurally.
Surgical technique
After being placed under general anaesthesia with double lumen endotracheal intubation, the patient is positioned in a lateral position. The surgeon stands anterior to the patient with the assistant and scrub nurse opposite to him, and the screen at the patient’s head. The incision is made on the anterior axillary line at either the 4th or 5th intercostal space according to whether the upper or lower lobe is involved, respectively. The length of the incision is about 3–4 cm. We used plastic wound protectors during in our study which can prevent camera polluted by blood from the wound.
The approximate position of the GGO can be calculated from preoperative CT images: the longitudinal position can be achieved by counting the slices above or below the GGO in a particular lobe. Moreover, the latitudinal position can be easily located by reading the corresponding projection on the body surface, such as scapular line, mid-axillary line, or mid-clavicular line (4).
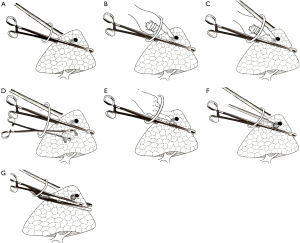
During the operation, the camera scope is always in the upper part of the port and the surgeon’s instruments in the lower part. The long oval forcep is then inserted from the bottom part of the port and massive lung tissue containing the nodule and the coil is lifted close to the incision to facilitate finger touch (Figure 2A). Then the camera scope is removed and the forefinger is dipped into the port. The technical key point to ensuring the position of the coil is the use of smooth long forceps as a solid background for finger touch. When the operator’s finger moves slowly forward along the instrument’s border, the coil on the border can then be easily felt as a protrusion (Figure 2B). After feeling the coil, the finger is kept still and the camera scope is reinserted to identify the finger’s position (Figure 2C). Another oval forcep is then inserted below the first forcep, and these functioning tissues are gently dragged back downward, making the coil protrusion more prominent and the covering pleura tighter (Figure 2D). The operator gently pushes the coil either left or right away from the oval forcep (Figure 2E). Such maneuver can then maintain about 1 cm of distance between the coil and the forcep. The pulmonary surface is then cauterized by the electrotome to mark the coil’s position (Figure 2F). A stapler is now inserted from the bottom of the port and placed below the forceps (Figure 2G). A wedge pulmonary resection can be made with a safe margin of at least 1.5 centimeters.
Postoperative outcomes
The operative time is 37.3±8.5 min (range, 20 to 50 min). Minimally invasive adenocarcinomas (MIA) were confirmed by frozen section in six cases. Adenocarcinoma in situ (AIS) was confirmed in three cases. Atypical adenomatous hyperplasia (AAH) was confirmed in three cases. One case was benign, which was fibrous tissue hyperplasia with carbon deposits. There was no 30-day mortality for any of the patients in this study. No patient had prolonged air leaks (>3 days after the operation). No major complications occurred. No regional recurrence was noticed at follow-up.
Comments
The localization of small pulmonary nodules, especially deeply located or in blind areas, is challenging in the uniportal VATS approach. Several methods for nodule location have been evaluated. All of these methods have their pros and cons. The practice of digital palpation is often inconclusive due to lack of a solid background and the interference of hilar structures. Guidance with use of methylene blue dye is limited by its potential to diffuse away from the nodule such that fixed time intervals between localization and surgical resection are required. Placement of a hookwire may cause tearing of the lung parenchyma with the potential for intrapulmonary hemorrhage and/or failed nodule localization if a pneumothorax develops. In addition, both hookwire and preoperative methylene blue injection have their blind areas.
Therefore, we aimed to combine radiologically guided microcoil localization with palpation by using a simple instrument like an oval forcep as a smooth background to discriminate, and then gradually narrowed the search range in a uniportal VATS operation. Compared with hook wire, our approach does not introduce pain caused by residual hook wire or injury to pulmonary tissue by dislodged anchoring hook and microcoil localization is feasible for lesions blocked by scapula and/or other biological structures. Compared with the traditional method of microcoil localization (5), there is no need to form a loop on the visceral pleural surface of the lung overlying the GGO, which is easier to operate while localizing the lesions. And we chose stainless microcoils instead of platinum ones since they can be felt as a protrusion more easily during palpation. Also, this technique may prevent medical staff and patients from radiation exposure during the surgery and has no special requirements for the operating room. This study was limited in that all GGO resections were performed at one academic teaching center by one surgeon (Chang Chen). Further experiences in other centers are required to determine whether this favorable surgical experience can be replicated.
For deeply located GGOs, many surgeons may choose segmentectomy or even lobectomy; however, if no diagnosis of invasive cancer has been obtained, wedge resection offers enhanced efficiency. The purpose of this study is just want to share our experience of wedge resection in uniportal VATS, provide another reliable surgical procedure. From our experience, a combination of CT-guided microcoil localization with palpation in uniportal VATS is a safe and effective procedure for accurate diagnosis and resection of indeterminate GGOs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Ethics Committee of Shanghai Pulmonary Hospital and written informed consent was obtained from all patients.
References
- Gonzalez D, de la Torre M, Paradela M, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg 2011;40:e21-8. [Crossref] [PubMed]
- Ng CS, Yeung EC, Wong RH, et al. Single-port sympathectomy for palmar hyperhidrosis with Vasoview Hemopro 2 endoscopic vein harvesting device. J Thorac Cardiovasc Surg 2012;144:1256-7. [Crossref] [PubMed]
- Chen YR, Yeow KM, Lee JY, et al. CT-guided hook wire localization of subpleural lung lesions for video-assisted thoracoscopic surgery (VATS). J Formos Med Assoc 2007;106:911-8. [Crossref] [PubMed]
- Zheng H, Jiang S, Chen C. Stepwise Tactile Localization and Wedge Resections for Deep Pulmonary Nodules during Video-Assisted Thoracoscopic Surgery. Thorac Cardiovasc Surg 2016;64:182-6. [Crossref] [PubMed]
- Finley RJ, Mayo JR, Grant K, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015;149:26-31. [Crossref] [PubMed]


