The relationship between preoperative serum cortisol level and the stability of plaque in carotid artery stenosis patients undergoing carotid endarterectomy
Introduction
Ischemic stroke is one of the worldwide leading causes of severe disability and mortality in ageing population (1,2). Currently, carotid luminal stenosis is the only acceptable diagnostic criterion for risk stratification of patients with carotid stenosis; however, growing evidence suggests that plaque stability may also represent a critical risk factor (1-4), as unstable plaque tends to rupture. Plague stability may result in severe neurological events for high-risk patients, and thus plaque stability should be considered in the clinical evaluations (5). Unstable plaque is characterized by a large lipid core, thin fibrous cap, intraplaque neovascularization, and a large inflammatory cell infiltrate composed of macrophages, T-cells and mast cell (6). However, stability of carotid plaque lacks of rapid examination target in serum, which is very important for screening. Therefore, discover of biological markers to evaluate stability of plaque is critically important (7).
Hypothalamic pituitary adrenal (HPA) axis plays an important role in physical functions by releasing cortisol which is implicated in the regulation of the inflammation system, lipid metabolism and glucose into the circulation. A dysfunctional HAP axis function has been described in several chronic inflammatory disorders, and can regulate mast cells function in allergic asthma (8). Thus HAP axis function is thought to be one of the possible mechanisms through psychosocial stress to influence the risk of atherosclerosis (9,10). Previous experimental works in animal models have found that atherosclerosis occurs in the context of elevated circulating cortisol level (11), but this finding requires further analysis (12,13). Moreover, it is still unclear whether serum cortisol influences the stability of carotid plaques.
The aim of this study was to investigate whether serum level of cortisol in patients with high-grade carotid stenosis could be useful to identify patients with unstable plaques. We tested the hypothesis that different level of preoperative serum cortisol may be linked to the different stability of carotid plaque.
Methods
Study population
Between May 2013 to October 2015, 101 patients with high-grade carotid stenosis who were undergoing carotid endarterectomy (CEA) in Vascular Surgery Department of Changhai Hospital were enrolled in our study. During that time, 28 patients were excluded including unavailable biological or histological samples, patients with chronic inflammatory/immunologic disorder, neoplasm disease, continuous treatment with immunosuppressive/anti-inflammatory agents, drug or alcohol abuse or poor mental function (14). Surgically removed carotid plaques were collected for histological analysis. This retrospective study was approved by the ethics committee of Changhai hospital.
Hormone analysis and laboratory measurements
Before the surgery, a fasting blood sample was taken for determination of biochemical parameters and cortisol in the morning at 8:00 AM. Plasma cortisol was measured by immunoassay (Unicel DxI 800,Beckman Coulter). The normal range of morning serum cortisol concentration in our hospital laboratory is 210 to 342 nmol/L.
Participants reported baseline characteristics, medical history and current smoking status. Height and weight were measured in light clothing for the calculation of body mass index (BMI). Degree of stenosis was measured by ultrasound. Serum total cholesterol and high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL), triglycerides (TG), fasting glucose, interleukin-6 (IL-6), white blood cell (WBC) and C reactive protein (CRP) were measured by routine assays in the hospital laboratory.
Histological method to define plaques stability
After the surgical samples removed, they were fixed in 10% neutral buffered formalin for 24 h, dehydrated in graded alcohols, cleared in xylene, and embedded in paraffin. Each sample was cut into 5 um and performs hematoxylin-and-eosin staining. According to the classification made by the American Heart Association identify the stable and unstable plaque (15). Type V lesion which formed with prominent new fibrous connective tissue together with multilayered lipid core is considered as stable plaque. Type VI lesion which have ulceration of endothelial disruption or intraplaque hemorrhage or thrombotic deposits is regarded as unstable plaque.
Statistical analysis
Categorical variables are reported as number (percent) and continuous variables as mean (SD) or median (25th to 75th interquartile range), depending on variable distribution. Group comparisons were analyzed with the Student t-test or Wilcoxon rank-sum test for numeric variables and the χ2 or Fisher exact test for categorical variables. Univariate analyses were used to assess the impact of post-operation laboratory examination index on the occurrence of adverse events for each patient. All analyses were performed using Empower (R) (www.empowerstats.com, X&Y solutions, inc., Boston, MA, USA) and R (http://www.R-project.org).
Results
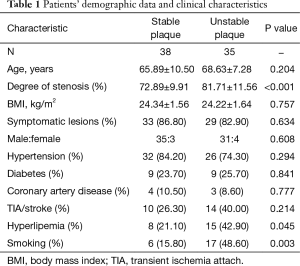
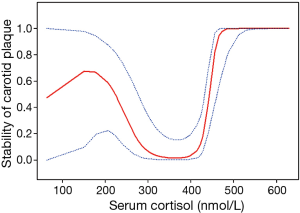
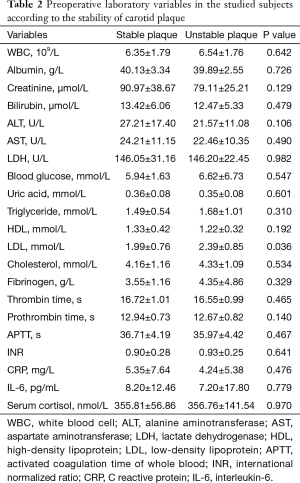
A total of 73 internal carotid stenosis patients undergoing CEA were enrolled in our study. Most patients were male (66 vs. 7). Unstable carotid plaque was detected in 35 (47.95%) subjects. Age distribution of the patients together with other risk factors is presented on Table 1 according to the stability of carotid plaque. Except for degree of stenosis (P<0.001), hyperlipemia (P=0.045) and smoking (P=0.003), there were no statistical differences in the demographic and clinical data between stable and unstable plaque groups. We used curve fitting method to find the relationship between preoperative serum cortisol and stability of carotid plaque and it represents a U-shape characteristic (Figure 1). The stability of carotid plaque of patients with low or high level of preoperative serum cortisol presents unstable status. The relationship between laboratory variables of interest and stability of carotid plaque is presented in Table 2. Only LDL can significantly promote the carotid plaque to an unstable status (P=0.036). Figure 2 showed the univariate analysis results of the risk factors which we thought might affect plaque stability. The degree of stenosis, hyperlipemia, smoking and LDL are the risk factors of carotid plaque stability.
Full table
Full table

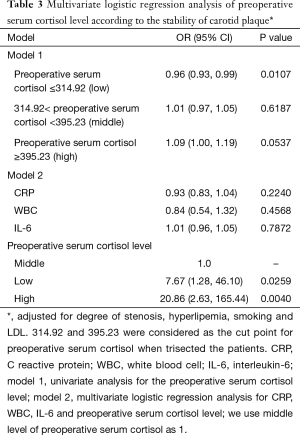
For further reveal the relationship between preoperative serum cortisol and the stability of carotid plaque, we divided preoperative serum cortisol into three levels equationally according to the numbers of patients. A total of 314.92 and 395.23 were considered as the cut point for preoperative serum cortisol. Univariate and multivariate logistic regression analysis were showed in Table 3 when adjusted for degree of stenosis, hyperlipemia, smoking and LDL. Model 1 is the univariate analysis result for the preoperative serum cortisol level. Model 2 is the multivariate logistic regression analysis for CRP, WBC, IL-6 and preoperative serum cortisol level. The odds ratio values of CRP, WBC and IL-6 are 0.93, 0.84 and 1.01 respectively. We use middle level of preoperative serum cortisol as model 1. It revealed that low and high level of preoperative serum cortisol can significantly affect carotid plaque to an unstable status when compared with the middle level. The odds ratio values of the low and high level of preoperative serum cortisol are 7.67 and 20.86 respectively.
Full table
Discussion
In this retrospective case-control study, our data suggests that preoperative serum cortisol has a relationship with carotid plaque stability. Carotid stenosis patients with low or high level of preoperative serum cortisol present more unstable carotid plaque than middle level patients. These results implicate the contributory role of cortisol to carotid atherosclerosis pathological process.
The HPA axis has a strong relationship with human’s health, and cortisol reflects HPA axis activity. HPA axis dysregulation is associated with rapid heart rate, hypertension, high level of cholesterol, LDL and fasting insulin (16,17). While extracranial arterial stenosis has been widely believe to contribute to the pathogenesis of stroke, more and more research has focused on the effects of cortisol on carotid diseases. In the Rotterdam Study, Dekker et al. (10) studied 1,866 participants of the Rotterdam Study who provided four salivary cortisol samples throughout on a single day. They found that total cortisol levels were associated with higher carotid plaque score in the elderly. Another study explored the relationship between women’s morning serum cortisol and coronary artery disease and revealed that increased cortisol levels might contribute to the occurrence of atherosclerosis (18). To our knowledge, more and more researches have found that stability of carotid has a close relationship with therapy method and prognosis on carotid stenosis (19). Our study focused on the regulatory role of serum cortisol on the stability of carotid plaque.
Many physiology mechanisms, such as inflammation, may explain the relationship between cortisol and the stability of atherosclerosis plaque. Inflammation plays an important role in the progression of atherosclerosis and cortisol has been widely used to regulate the inflammatory system especially as it relates to controlling vessel walls during an inflammatory response (20,21). In fact many inflammatory factors have already been described as markers of carotid stenosis risk (22). For example, high hsCRP levels are associated with an increased embolization in carotid artery stenting (23). Morever, Grönberg et al.’s research found that high levels of IL-16 are associated with expression of factors contributing to plaque stability and decreased risk of new cardiovascular events during a 2-year period following surgery (24). The clinical significance of macrophage phenotypes in carotid plaque stability has also been widely studied (25). Fittipaldi et al. found that higher levels of CRP and vascular-endothelial-growth-factor (VEGF) correlated with unstable plaque (26). These results indicate that inflammation is an important factor in regulating carotid plaque stability. Although our study didn’t identify a significant influence of serum inflammatory indicators such as CRP and IL-6 on carotid plaque stability, we can not neglect the contributions of inflammation. HPA axis might control the expression level of serum inflammatory indicators. The relationship between serum cortisol and the inflammation indicators according to the stability of carotid plaque stability needs to be further researched.
Besides that, physiological stress may also affect endocrine responses (27). Thus, we considered that physiological stress may contribute to cardiovascular disease through regulating HPA axis. Reportedly, repeated episodes of acute stress can accelerate the speed of the inflammatory response in vessel wall (28). Moreover, Barnett et al. (29) found that reactivity to physiological stress may influence the progression of atherosclerosis in carotid artery disease. We believe that physiological stress, namely cortisol levels, may also affect the stability of carotid plaques in patients with carotid stenosis. However, the mechanism by which serum cortisol affects carotid plaque stability need to be further studied.
Limitation
The results of our study may be limited by the relative small number of subjects and lack of postoperative follow-up. Thus, more high-quality, multiple-center, large-sample randomized study are required to further verify our study. In addition, the normal level of cortisol presents fluctuate pattern in a day, which is in highest point at about 30 minutes after wake up in the morning and in the lowest point at midnight. We just exam the preoperative serum cortisol level in the morning, regardless of fluctuation of cortisol which need multi-time exam to evaluate in 1 day. However, it is difficult to obtain blood samples in 1 day at several different times. In the future study, we could use salivary sample instead of serum sample to overcome this problems as a result of salivary cortisol level have a high accuracy reflection of serum cortisol level.
Conclusions
In summary, our data clearly demonstrate that the relationship between preoperative serum cortisol and carotid plaque stability in carotid stenosis patients undergoing CEA. Low or high levels of preoperative serum cortisol might be an adverse factor to carotid plaque stability. Maintaining the serum cortisol level to a middle level may benefit for carotid stenosis patients clinical treatment. At the same time, serum cortisol can be used as a biological marker to assess the stability of carotid plaque.
Acknowledgements
Funding: This study was funded by the National Natural Science Foundation of China (81330034 and 81273522).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the ethics committee of Changhai hospital.
References
- Virmani R, Burke AP, Kolodgie FD, et al. Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. J Interv Cardiol 2003;16:267-72. [Crossref] [PubMed]
- Zhang J, Xu R, Liu P, et al. Prevalence of carotid artery stenosis in Chinese patients with angina pectoris. J Thorac Dis 2015;7:2300-6. [PubMed]
- Ren S, Fan X, Peng L, et al. Expression of NF-κB, CD68 and CD105 in carotid atherosclerotic plaque. J Thorac Dis 2013;5:771-6. [PubMed]
- van Lammeren GW, Reichmann BL, Moll FL, et al. Atherosclerotic plaque vulnerability as an explanation for the increased risk of stroke in elderly undergoing carotid artery stenting. Stroke 2011;42:2550-5. [Crossref] [PubMed]
- Naylor AR. Time to rethink management strategies in asymptomatic carotid artery disease. Nat Rev Cardiol 2011;9:116-24. [Crossref] [PubMed]
- Libby P. Inflammation in atherosclerosis. Nature 2002;420:868-74. [Crossref] [PubMed]
- Shah PK. Biomarkers of plaque instability. Curr Cardiol Rep 2014;16:547. [Crossref] [PubMed]
- Zhou J, Liu DF, Liu C, et al. Glucocorticoids inhibit degranulation of mast cells in allergic asthma via nongenomic mechanism. Allergy 2008;63:1177-85. [Crossref] [PubMed]
- Nijm J, Jonasson L. Inflammation and cortisol response in coronary artery disease. Ann Med 2009;41:224-33. [Crossref] [PubMed]
- Dekker MJ, Koper JW, van Aken MO, et al. Salivary cortisol is related to atherosclerosis of carotid arteries. J Clin Endocrinol Metab 2008;93:3741-7. [Crossref] [PubMed]
- de Prada TP, Pozzi AO, Coronado MT, et al. Atherogenesis takes place in cholesterol-fed rabbits when circulating concentrations of endogenous cortisol are increased and inflammation suppressed. Atherosclerosis 2007;191:333-9. [Crossref] [PubMed]
- Troxler RG, Sprague EA, Albanese RA, et al. The association of elevated plasma cortisol and early atherosclerosis as demonstrated by coronary angiography. Atherosclerosis 1977;26:151-62. [Crossref] [PubMed]
- Koertge J, Al-Khalili F, Ahnve S, et al. Cortisol and vital exhaustion in relation to significant coronary artery stenosis in middle-aged women with acute coronary syndrome. Psychoneuroendocrinology 2002;27:893-906. [Crossref] [PubMed]
- Nijm J, Kristenson M, Olsson AG, et al. Impaired cortisol response to acute stressors in patients with coronary disease. Implications for inflammatory activity. J Intern Med 2007;262:375-84. [Crossref] [PubMed]
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995;15:1512-31. [Crossref] [PubMed]
- Walker BR, Soderberg S, Lindahl B, et al. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J Intern Med 2000;247:198-204. [Crossref] [PubMed]
- Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab 1998;83:1853-9. [PubMed]
- von Känel R, Mausbach BT, Kudielka BM, et al. Relation of morning serum cortisol to prothrombotic activity in women with stable coronary artery disease. J Thromb Thrombolysis 2008;25:165-72. [Crossref] [PubMed]
- Sannino A, Brevetti L, Giugliano G, et al. Non-invasive vulnerable plaque imaging: how do we know that treatment works? Eur Heart J Cardiovasc Imaging 2014;15:1194-202. [Crossref] [PubMed]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol 2002;21:531-41. [Crossref] [PubMed]
- Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol 2007;157:545-59. [Crossref] [PubMed]
- Pini R, Faggioli G, Fittipaldi S, et al. Inflammatory mediators and cerebral embolism in carotid stenting: new markers of risk. J Endovasc Ther 2013;20:684-94. [Crossref] [PubMed]
- Faggioli G, Fittipaldi S, Pini R, et al. C-reactive protein and embolization during carotid artery stenting. A serological and morphological study. Histol Histopathol 2011;26:843-53. [PubMed]
- Grönberg C, Bengtsson E, Fredrikson GN, et al. Human Carotid Plaques With High Levels of Interleukin-16 Are Associated With Reduced Risk for Cardiovascular Events. Stroke 2015;46:2748-54. [Crossref] [PubMed]
- Medbury HJ, Williams H, Fletcher JP. Clinical significance of macrophage phenotypes in cardiovascular disease. Clin Transl Med 2014;3:63. [Crossref] [PubMed]
- Fittipaldi S, Pini R, Pasquinelli GA, et al. High Sensitivity C-Reactive Protein and Vascular Endothelial Growth Factor as Indicators of Carotid Plaque Vulnerability. J Cardiovasc Surg (Torino) 2014. [Epub ahead of print]. [PubMed]
- Spence JD, Munoz C, Huff MW, et al. Effect of amlodipine on hemodynamic and endocrine responses to mental stress. Am J Hypertens 2000;13:518-22. [Crossref] [PubMed]
- Hamer M, Endrighi R, Venuraju SM, et al. Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS One 2012;7:e31356. [Crossref] [PubMed]
- Barnett PA, Spence JD, Manuck SB, et al. Psychological stress and the progression of carotid artery disease. J Hypertens 1997;15:49-55. [Crossref] [PubMed]


