Long-term performance of the second-generation cobalt-chromium sirolimus-eluting stents in real-world clinical practice: 3-year clinical outcomes from the prospective multicenter FOCUS registry
Introduction
Drug-eluting stents (DES), initially designed to inhibit neointimal proliferation, have proved highly effective in reducing the incidence of stent failure and thus been broadly accepted as standard care of coronary artery disease in interventional cardiovascular community (1-3). The first-generation DESs, Cypher sirolimus-eluting stent (SES) and Taxus paclitaxel-eluting stent (PES), have been demonstrated to dramatically reduce the incidence of restenosis and thus significantly abate the need for target lesion revascularization (TLR) compared with bare metal stents (4-7). However, stent thrombosis have been raised as another challenging issue about the first-generation stents and are widely considered as a significant hazards necessitating longer duration of dual antiplatelet therapy (DAPT) (8-10). These limitations of the first-generation DESs spurred the innovation of stent technology. As a result, the second-generation DESs such as Endeavor zotarolimus-eluting stent (ZES) and Xience V/Promus everolimus-eluting stent (EES) and newer second-generation DESs such as Xience Prime EES and Resolute ZES have been produced. With the stent platform revolution from stainless steel to cobalt chromium or platinum chromium, the second-generation DESs are characterized by thinner stent struts that are supposed to attenuate acute vessel injury and thus alleviate local inflammatory response and therefore decrease the incidence of adverse events related to implanted stents. As the second-generation DESs were widely applied to clinical settings in the past decades, a number of clinical trials and real-world registries have been conducted to evaluate the safety and effectiveness of the second-generation DESs and its superiority to the first-generation DESs, whereas, the results seem controversial (11-18).
The Firebird SES, made by MicroPort medical, Shanghai, China, was a first-generation DES widely used in Asian countries, especially in China. Multiple randomized clinical trials and real-world registries have shown that Firebird SES was able to significantly improve the clinical and angiographic outcomes after stent implantation (19-21). With the evolution of stent techniques, the Firebird-2 cobalt-chromium SES (Firebird-2 CoCr-SES) with the characteristics of reduced strut thickness, enhanced radiopacity, improved deliverability, and higher biocompatibility was produced and permitted for use in clinical settings based on the safe and effective clinical outcomes observed in randomized clinical trials comparing Firebird-2 SES with its bare metal counterpart in patients with relatively simple coronary lesions (22). Therefore, the Firebird-2 cObalt-Chromium alloy sirolimus-elUting Stent registry (FOCUS registry) was initiated to evaluate the safety and efficacy of the Firebird-2 CoCr-SES in real-world patients.
From March 2009 to February 2010, a total of 5,084 consecutive patients were enrolled into the FOCUS registry from 83 participating centers in China, Thailand and Indonesia. The short- and mid-term clinical outcomes had been published previously (23-25). This article is aim at reporting the safety and effectiveness of the Firebird-2 CoCr-SES in relatively unselected population of patients at 3 years and discussing the long-term performance of the second-generation CoCr-SES in real-world clinical practice in Asian countries.
Methods
Study design and objectives
A detailed description of the FOCUS registry has been published previously (23-25). In brief, the FOCUS registry was a large-scale, prospective, single-arm post-market surveillance study involving 83 clinical centers in three Asian countries (China, Thailand, and Indonesia). A total of 5,084 patients eligible to receive the second-generation CoCr-SES were enrolled consecutively from March 2009 to February 2010. The objective of this study was to assess the safety and effectiveness of the Firebird-2 CoCr-SES in real-world patients requiring stent implantation.
The study was conducted in conformity with the ethic guidelines of the Declaration of Helsinki. Prior to study initiation, the registry was approved by the Research Ethics Committee at each participating clinical centers depending on regional requirements. Written informed consents were obtained from all participating subjects or their legal relatives.
Study population and protocol
All patients with single or multi-vessel lesions appropriate to receive Firebird-2 CoCr-SES except those presenting with myocardial infarction (MI) within 72 hours were eligible for enrollment in the FOCUS registry. Thereby, the study population included real-world patients with severe complications and complex lesions who were usually excluded in randomized clinical trials. Enrolled subjects were subdivided into standard-use group and extended-use group. Definition of extended use and standard use was described in previously published literature (24). In brief, the extended-use group was defined as patients with left main lesions, chronic total occlusions, bypass graft lesions, in-stent restenosis, bifurcated or ostial lesions, severe tortuosity, multi-vessel stenting, severe calcification, reference diameter <2.5 mm, lesion length >28 mm, or moderate or severe renal impairment. All other patients were classified as standard-use.
All recruited patients were prescribed with antiplatelet therapy before the procedure of percutaneous coronary intervention (PCI) according to the standard care of each center. Procedures and visual estimation of lesion characteristics were performed by experienced senior interventional cardiologists. One or more Firebird-2 CoCr-SES was allowed to be implanted into the target vessels according to the interventional clinicians’ discretions and if two or more stents are required during the procedure, Firebird-2 CoCr-SES should be the exclusive option. DAPT with clopidogrel 75 mg and aspirin 100 mg per day was required to last for at least 12 months for all subjects and aspirin was required indefinitely after the end of DAPT. The Follow-up of the study was conducted by telephone interview or hospital visit. Angiographic follow-up was not mandatory in the FOCUS registry protocol.
Study endpoints
The primary endpoints of 3-year follow-up included MACE and TLF. MACE was a composite endpoint of cardiac death, non-fatal MI and TVR. TLF was a composite endpoint of cardiac death, MI related to the target vessels and a clinically indicated TLR. The secondary endpoints included each individual component of the primary endpoints, all-cause death and ARC definite/probable stent thrombosis. The detailed definitions of each endpoint were described elsewhere (23).
Data collection and management
Data of each patient and characteristics of the lesions including the location of the target vessel, the ACC/AHA defined lesion type, the visually-estimated reference vessel diameter (RVD), lesion length and lesion diameter stenosis were reported to an independent clinical endpoint committee consisting of experienced cardiologists not participating in the study in a web-based manner. All events related to endpoints were adjudicated by an independent clinical endpoints committee to ensure the accuracy of data. Each selected center was randomly monitored to detect and correct any inaccuracy of the recorded data and to check for under-reporting of events. All these measures ensured high quality of the FOCUS registry and enhanced the validity of the data reported in this paper.
Statistical analysis
Baseline data of the patients’ demographics, lesion characteristics and safety and efficacy endpoints of all enrolled subjects were summarized and described as descriptive statistics. Continuous variables are presented as mean ± standard deviation (SD) and Categorical variables as percentage. Independent sample t-test was used for comparison of mean values between extended-use group and standard-use group and chi-square test or Fisher’s exact test for proportions. The time to MACE event, TLF event and stent thrombosis were analyzed and demonstrated with cumulative incidence curve via Kaplan-Meier method.
Results
Patient’s demographics and characteristics
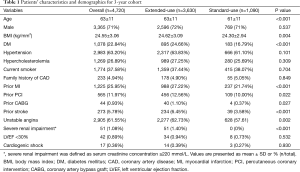
A total of 4,720 (92.8%) of the initially enrolled 5,084 patients were available for 3-year follow-up including 3,630 patients in extended-use group and 1,090 patients in standard-use group. The overall mean age was 63±11. Among them, 71% were males. The proportions of patients with diabetes mellitus, prior MI, unstable angina, severe renal failure or prior stroke were significantly higher in extended-use group than that in standard-use group. In addition, there were more patients in the extended-use group who had history of prior PCI or CABG. Other detailed information of the patient characteristics was shown in Table 1.
Full table
Lesion characteristics
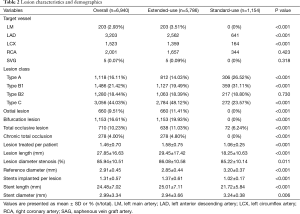
A total of 6,940 lesions were treated in the available 4,720 patients. The mean target lesion length was 27.85±16.63 mm and the mean target lesion diameter stenosis was 86%±11%. More lesions in extended-use group belonged to type B2/C and the proportion of complex lesions such as ostial lesion, bifurcation lesion, and total occlusive lesion was significantly higher in extended-use group. In a word, lesions treated in extended-use group were more complex than those treated in standard-use group. Accordingly, the stents required in extended-use group were longer in length and smaller in diameter. Other lesion characteristics are illustrated in Table 2.
Full table
Overall clinical outcomes
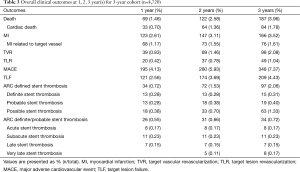
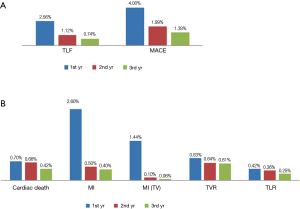
Three-year follow-up data was acquired from 4,720 (92.8%) of 5,084 initially enrolled patients. The incidence of MACE at 3 years was 7.37%, including 1.78% (84 cases) cardiac death, 3.52% (166 cases) non-fatal MI and 2.08% (98 cases) TVR as demonstrated in Table 3. According to the dynamic change of each endpoint of the available 4,720 patients at 1, 2, 3 year(s) summarized in Table 3, the incidence of adverse clinical events increased incrementally. The rate of MACE increased from 4.13% at 1 year to 5.93% at 2 years and remained low (7.37%) at 3 years. Meanwhile, the rate of TLF at 1 year (2.56%), 2 years (3.69%) and 3 years (4.43%) increased modestly and remained in a very low rate.
Full table
In addition, adverse clinical events are mainly reported at the first year after initial procedure as demonstrated in Figure 1A. The rate of MACE and TLF reported in the second year (1.99% and 1.12%) and the third year (1.38% and 0.74%) reduced gradually. Notably, MI and MI related to target vessels are predominantly happened in the first year (2.60% and 1.44%) after stent implantation. Only 24 (0.50%) cases of MI and 5 (0.11%) cases of MI related to target vessel were reported in the second year and the cases reduced even lower to 19 (0.40%) and 3 (<0.10%) in the third year. Other adverse events distributed evenly in the 1st, 2nd, 3rd year but also showed a modestly reduced trend (Figure 1B).
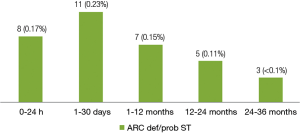
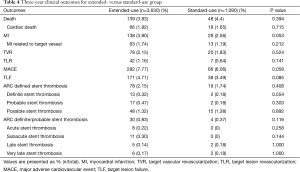
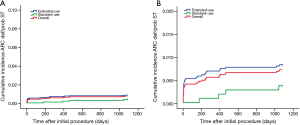
A total of 34 (0.72%) cases of ARC definite/probable stent thrombosis were reported up to 3 years, including 8 (0.17%) cases of acute stent thrombosis, 11 (0.23%) cases of subacute stent thrombosis, 7 (0.15%) cases of late stent thrombosis and 8 (0.17%) cases of very late stent thrombosis. The number of very late stent thrombosis reported in the second year and the third year were 5 (0.11%) and 3 (<0.1%) respectively. It means stent thrombosis mainly happened in the first year after stent implantation, thereafter, the incidence of stent thrombosis reduced remarkably to a very low level (Figure 2).
Clinical outcomes for standard-use group versus extended-use group
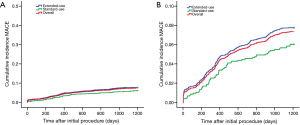
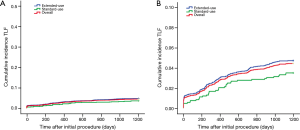
Although the extended-use group included more patients with severe complications and lesions treated in extended-use group were more complicated compared with standard-use group (shown in Tables 1,2), the rates of MACE (7.77% vs. 6.06%, P=0.058), TLF (4.71% vs. 3.49%; P=0.085) and ARC definite/probable stent thrombosis (0.83% vs. 0.37%; P=0.116) were not significantly increased in extended-use group, so did the incidence of each individual component of MACE and TLF (shown in Table 4). Cumulative incidence of MACE, TLF and ARC definite/probable stent thrombosis up to 3 years were also not significantly different between two groups (shown in Figures 3-5).
Full table
Discussion
As we know, the second-generation DESs are mainly characterized by the innovation of stent platform and the eluting drugs. Similar to most second-generation DESs, the stent platform of the Firebird-2 stents was made of cobalt-chromium, but the eluting drug remains sirolimus instead of its derivatives. Although, lots of international registries aimed at evaluating the safety and effectiveness of the second generation DES like EES or ZES have been conducted to date. However, none of these registries has focused on the Firebird 2 stents that are broadly applied to domestic clinical settings. The existing domestic studies on this topic are either limited to size or confined to less complicated populations. The present study is a comprehensive registry enrolling almost all-comers and thus will more convincing to reflect the real-life clinical practice. Therefore, the superiority of the Firebird-2 CoCr-SES to the first-generation SES and the non-inferiority to other types of second-generation DESs were two main issues of the FOCUS registry.
According to the 3-year outcomes from REWARDS registry comparing sirolimus- and PESs in an unselected population with coronary artery disease, the rates of MACE (28.1%) and stent thrombosis (2.2%) of first-generation SES were much higher than those of the Firebird-2 CoCr-SES observed in the FOCUS registry (26). This indicated an obvious superiority of the Firebird-2 CoCr-SES to the first-generation SES. Meanwhile, the rate of 3-year TLF (4.43%) observed in our FOCUS registry was comparable to those reported in the 3-year outcomes from the multicenter prospective EXCELLENT and RESOLUTE-Korean registries comparing the second-generation everolimus-eluting Xience V stents and zotarolimus-eluting resolute stents. The rate of TLF at 3-year follow-up for Resolute ZES and Xience V EES was 6.4% and 6.2% respectively suggesting that Firebird-2 CoCr-SES was not inferior to other types of second-generation DES (27).
Additionally, the FOCUS registry showed a very low rate of ARC definite/probable stent thrombosis at 2 and 3 years, comparable to 2-year follow-up results in E-FIVE registry evaluating the performance of the Endeavor ZES and 3-year follow-up in EXCELLENT and RESOLUTE registry (28). Overall, The incidence of very late stent thrombosis in the present report at 2 and 3 years (0.11% and 0.17% respectively) was similar with that reported in previously published literatures (27,28). More specifically, 3-year clinical outcomes from the multicenter prospective EXCELLENT and RESOLUTE registries demonstrated 4 cases of very late stent thrombosis in total 5,054 patients including 3 cases in EES subgroup (n=3,056) and 1 case in ZES subgroup (n=1,998) (27). Similarly, only 3 (<0.1%) new cases of very late stent thrombosis were reported in the third year of the FOCUS study.
In conclusion, this report presents the detailed 3-year follow-up data from the large prospective registry of Firebird-2 CoCr-SES in real-world clinical practice. The clinical outcomes in 3 consecutive years and the distribution of each endpoint in very separate year were summarized to provide a vivid insight into the dynamic changes of each endpoint of safety and efficacy in 3 years. In accordance with the results at 12-month and 2-year follow-up, the 3-year data were promising for the Firebird-2 CoCr-SES despite the high proportion of enrolled patients with high-risk factors and complicated lesions who were usually not included in the randomized clinical trials. Notably, the results from the FOCUS registry are similar to those previously published from other multicenter prospective registries, therefore, can be taken as a potent evidence to support the safety and effectiveness of the Firebird-2 CoCr-SES in real-world patients.
Limitation
The present study has several limitations. First, the fact that 7.2% [364] of the initially enrolled patients had been lost at 3-year follow-up should be acknowledged as a main factor affecting the results of the 3-year analysis of the safety endpoints. Second, acute MI within 72 hours was excluded in our FOCUS study, which may be the reason for a better clinical outcome observed in our study. Third, intrinsic limitation of nonrandomized study should be considered when interpreting the results of the study. Last, intravascular imaging technologies like intravascular ultrasound and optical coherence tomography are of great importance for optimizing the stenting strategy, however, patients’ information about the intravascular imaging was not completely documented in the present study. This may partially influence the overall evaluation of the performance of the CoCr-SES.
Conclusions
Extended 3-year follow-up of this large cohort of patients from the FOCUS registry further confirmed the long-term safety and effectiveness of the second-generation CoCr-SES in daily clinical practice.
Acknowledgements
Funding: The FOCUS registry was sponsored and partially founded by Shanghai MicroPort Medical (Group) Co., Ltd. This work was also supported by the National Natural Science Foundation (No. 81101133) and the Science and Technology Cooperation Foundation from the Shanghai Science and Technology Development (No. 14695840800).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The registry was approved by the Research Ethics Committee at each participating clinical centers depending on regional requirements. Written informed consents were obtained from all participating subjects or their legal relatives.
Supplementary
Participating centers and principal investigators of the FOCUS registry were as follows (Sequence according to the contributed case numbers): Zhongshan Hospital of Fudan University (Dr. Junbo Ge), Fuwai Cardiovascular Hospital (Dr. Yuejin Yang), Beijing Anzhen Hospital (Dr. Fang Chen, Xiaoling Zhu, Shuzheng Lv, Zhizhong Li), Xi’an Xijing Hospital (Dr. Haichang Wang), Tianjin Chest Hospital (Dr. Yin Liu), Shanghai Tenth People’s Hospital (Dr. Yawei Xu), The People’s Hospital of Liaoning Province (Dr. Zhanquan Li), Putuo District Certral Hospital of Shanghai (Dr. Huigen Jin), Shanghai Chest Hospital (Dr. Weiyi Fang), The First Affiliated Hospital of Dalian Medical University (Dr. Xuchen Zhou), Guangdong General Hospital (Dr. Lijun Jing), People’s Liberation Army General Hospital (Dr. Yundai Chen), The Affiliated Hospital of Medical College of Qingdao University (Dr. Changyong Zhou, Zhexun Lian, Yi An), Chengdu Military General Hospital (Dr. Yongjian Yang), Meizhou people’s hospital (Dr. Zhixiong Zhong), The First Affiliated Hospital of Medical College of Xi’an Jiaotong University (Dr. Zuyi Yuan), The First People’s Hospital of Jining City (Dr. Xiaofei Sun), Jiangxi Provincial People’s Hospital (Dr. Guotai Sheng), General Hospital of Fushun Mining Group (Dr. Zhenming Yan, RenKe Yi), Shanxi Provincial Institute of Cardiovascular Disease (Dr. Jian An), The Second Hospital of Jilin University (Dr. Bin Liu), Cangzhou Central Hospital (Dr. Zesheng Xu), Xiangya Hospital of Zhongnan University (Dr. Xiaoqun Pu), Shanghai Changhai Hospital (Dr. Yongwen Qin), Xingtai Cardiovascular Disease Hospital (Dr. Shuangying Feng), Sir Run Run Shaw Hospital of Zhejiang University (Dr. Guosheng Fu), The People’s Hospital of Shanxi Province (Dr. Chunlin Lai), The Second Clinical College of Harbin Medical University (Dr. Bo Yu), Armed Police Medical College Hospital (Dr. Tiemin Jiang), Beijing Chaoyang Hospital (Dr. Lefeng Wang), People’s Hospital of Peking University (Dr. Weiming Wang), Beijing Shijitan Hospital (Dr. Jianjun Peng), Daqing Oil Field General Hospital (Dr. Hui Li), Shengjing Hospital of China Medical University (Dr. Wenyue Pang), Nanjing Drum Tower Hospital (Dr. Biao Xu), Huashan Hospital of Fudan University (Dr. Haiming Shi), Shanghai Sixth People’s Hospital (Dr. Meng Wei), Shanghai Xinhua Hospital (Dr. Changqian Wang), The Second Affiliated Hospital of Zhejiang University (Dr. Ying Zhu), Hangzhou First People’s Hospital (Dr. Ningfu Wang), Nanjing First Hospital (Dr. Shaoliang Chen), Xuzhou Centre Hospital (Dr. Qiang Fu), Affiliated Hospital of Guangdong Medical College (Dr. Shian Huang, Keng Wu), Weifang Yidu Zhongxin Hospital (Dr. Yutian Tong), The First Affiliated Hopital of Lanzhou University (Dr. Zheng Zhang), The Second Affiliated Hospital of Kunming Medical College (Dr. Ge Zhang), Wuhan Tongji Hospital of Huazhong University of Science and Technology (Dr. Hesong Zeng), Wuhan University Renmin Hospital (Dr. Hong Jiang), Xiangfan City Central Hospital (Dr. Wenwei Liu), Wuhan Asia Heart Hospital (Dr. Guoying Zhu), First People’s Hospital of Yulin City (Dr. Ping Li), Beijing Luhe Hospital (Dr. Xuekun Zhang), The First Affiliated Hospital of Sun yatsen University (Dr. Zhimin Du), Zhujiang Hospital (Dr. Yingfeng Liu), The First Clinical College of Harbin Medical University (Dr. Weimin Li), The people’s hospital of Guangxi Zhuang Autonomous Region (Dr. Ling Liu), Shanghai Renji Hospital (Dr. Ben He), The Third Hospital of Peking University (Dr. Wei Gao), The first Affiliated Hospital of China Medical University (Dr. Dalin Jia), Affiliated Hospital of Jiangsu University (Dr. Jinchuan Yan), Yantai Yuhuangding Hospital (Dr. Shaorong Liu, Chuanhuan Zhang, Yimin Fang, Zhigang Tao), Qilu Hospital of Shandong University (Dr. Jifu Li), Xiangtan Central Hospital (Dr. He Huang), Shanghai Huadong Hospital(Dr. Xingui Guo), Shandong Provincial Hospital (Dr. Xinghua Zhang, Tongbao Liu, Lianqun Cui), The First Affiliated Hospital of Kunming Medical College (Dr. Jianming Xiao), Fujian Medical University Union Hospital (Dr. Lianglong Chen), Tongji Hospital of Tongji University (Dr. Jinfa Jiang), Tianjin First Central Hospital (Dr. Chenzhi Lu), The Fourth Clinical College of Harbin Medical University (Dr. Xueqi Li), The Second Affiliated Hospital of Dalian Medical University (Dr. Peng Qu), Zhongshan Hospital of Xiamen University (Dr. Yan Wang), The First People’s Hospital of Ningbo City (Dr. Honghua Ye), Affiliated Hospital of Weifang Medical College (Dr. Jingtian Li), Guangzhou Nanfang Hospital, Southern Medical University (Dr. Yuqing Hou), Wuhan Union Hospital(Dr. Qiutang Zeng), The Fourth Affiliated Hospital of China Medical University (Dr. Yuanze Jin), Dalian 210 Hospital (Dr. Dongju Jiang), The First Affiliated Hospital of Chongqing Medical University (Dr. Kanghua Ma), Shanghai Minhang District Certral Hospital (Dr. Dadong Zhang), Siriraj Hospital of Mahidol University, Bangkok, Thailand (Dr. Suwatchai Pornratanarangsi), Bina Waluya Cardiac Hospital, Indonesia (Dr. Muhamad Munawar), Medistra Hospital, Indonesia (Dr. Teguh Santoso).
References
- Katz G, Harchandani B, Shah B. Drug-eluting stents: the past, present, and future. Curr Atheroscler Rep 2015;17:485. [Crossref] [PubMed]
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur Heart J 2015;36:3320-31. [Crossref] [PubMed]
- Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002;346:1773-80. [Crossref] [PubMed]
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315-23. [Crossref] [PubMed]
- Weisz G, Leon MB, Holmes DR Jr, et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) Trial. J Am Coll Cardiol 2009;53:1488-97. [Crossref] [PubMed]
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004;350:221-31. [Crossref] [PubMed]
- Ellis SG, Stone GW, Cox DA, et al. Long-term safety and efficacy with paclitaxel-eluting stents: 5-year final results of the TAXUS IV clinical trial (TAXUS IV-SR: Treatment of De Novo Coronary Disease Using a Single Paclitaxel-Eluting Stent). JACC Cardiovasc Interv 2009;2:1248-59. [Crossref] [PubMed]
- Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 2007;369:667-78. [Crossref] [PubMed]
- Franck C, Eisenberg MJ, Dourian T, et al. Very late stent thrombosis in patients with first-generation drug-eluting stents: a systematic review of reported cases. Int J Cardiol 2014;177:1056-8. [Crossref] [PubMed]
- Montalescot G, Brieger D, Dalby AJ, et al. Duration of Dual Antiplatelet Therapy After Coronary Stenting: A Review of the Evidence. J Am Coll Cardiol 2015;66:832-47. [Crossref] [PubMed]
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med 2010;362:1663-74. [Crossref] [PubMed]
- Stone GW, Rizvi A, Sudhir K, et al. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. J Am Coll Cardiol 2011;58:19-25. [Crossref] [PubMed]
- Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 2010;375:201-9. [Crossref] [PubMed]
- Kimura T, Morimoto T, Natsuaki M, et al. Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-eluting Versus Everolimus-eluting stent Trial (RESET). Circulation 2012;126:1225-36. [Crossref] [PubMed]
- Jensen LO, Thayssen P, Hansen HS, et al. Randomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV). Circulation 2012;125:1246-55. [Crossref] [PubMed]
- Räber L, Jüni P, Nüesch E, et al. Long-term comparison of everolimus-eluting and sirolimus-eluting stents for coronary revascularization. J Am Coll Cardiol 2011;57:2143-51. [Crossref] [PubMed]
- Kandzari DE, Leon MB, Popma JJ, et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J Am Coll Cardiol 2006;48:2440-7. [Crossref] [PubMed]
- Eisenstein EL, Leon MB, Kandzari DE, et al. Long-term clinical and economic analysis of the Endeavor zotarolimus-eluting stent versus the cypher sirolimus-eluting stent: 3-year results from the ENDEAVOR III trial (Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovasc Interv 2009;2:1199-207. [Crossref] [PubMed]
- Liu HB, Xu B, Gao RL, et al. Outcomes of using Firebird rapamycin eluting stents in routine coronary intervention practice: one-year results from the pilot study of Firebird in China registry. Chin Med J (Engl) 2006;119:609-11. Long-term efficacy and safety of Chinese made sirolimus eluting stents: results, including off label usage, from two centres over three years. [PubMed]
- Tresukosol D, Pornratanarangsi S, Chotinaiwattarakul C, et al. Evaluation of the firebird sirololimus eluting stent in all comers with coronary artery stenosis. J Med Assoc Thai 2010;93:S11-20. [PubMed]
- Zhang Q, Xu B, Yang YJ, et al. Long-term efficacy and safety of Chinese made sirolimus eluting stents: results, including off label usage, from two centres over three years. Chin Med J (Engl) 2008;121:1670-4. [PubMed]
- Zhang Q, Xu B, Yang YJ, et al. Sirolimus-eluting cobalt alloyed stents in treating patients with coronary artery disease: six-month angiographic and one-year clinical follow-up result. A prospective, historically controlled, multi-center clinical study. Chin Med J (Engl) 2007;120:533-8. [PubMed]
- Ge JB, Zhang F, Qian JY, et al. Six-month clinical outcomes of Firebird 2TM sirolimus-eluting stent implantation in real-world patients with coronary artery diseases. Chin Med J (Engl) 2011;124:831-5. [PubMed]
- Zhang F, Ge J, Qian J, et al. Real-world use of the second-generation cobalt-chromium sirolimus-eluting stents: 12-month results from the prospective multicentre FOCUS registry. EuroIntervention 2012;8:896-903. [Crossref] [PubMed]
- Zhang F, Ge J, Qian J, et al. Two-year clinical outcomes of patients with the second-generation cobalt-chromium sirolimus-eluting stents from the real-world FOCUS registry. Int J Cardiol 2013;166:750-2. [Crossref] [PubMed]
- Hanna NN, Gaglia MA Jr, Torguson R, et al. Three-year outcomes following sirolimus- versus paclitaxel-eluting stent implantation in an unselected population with coronary artery disease (from the REWARDS Registry). Am J Cardiol 2010;106:504-10. [Crossref] [PubMed]
- Lee JM, Park KW, Han JK, et al. Three-year patient-related and stent-related outcomes of second-generation everolimus-eluting Xience V stents versus zotarolimus-eluting resolute stents in real-world practice (from the Multicenter Prospective EXCELLENT and RESOLUTE-Korea Registries). Am J Cardiol 2014;114:1329-38. [Crossref] [PubMed]
- Meredith I, Rothman M, Erglis A, et al. Extended follow-up safety and effectiveness of the Endeavor zotarolimus-eluting stent in real-world clinical practice: two-year follow-up from the E-Five Registry. Catheter Cardiovasc Interv 2011;77:993-1000. [Crossref] [PubMed]