Transition to routine use of venoarterial extracorporeal oxygenation during lung transplantation could improve early outcomes
Introduction
Cardiopulmonary bypass (CPB) has been the standard modality used for intraoperative cardiorespiratory support during lung transplantation (LTx). However, there is much debate on the use of CPB during LTx (1,2). Some authors recommend routine use of CPB (1) because it provides optimal intraoperative hemodynamic stability and controls low-pressure reperfusion. So, it protects lung and other organs from damage and allows good hilar exposure during operation. However, others do not recommend that the routine use of CPB (2). The use of CPB is associated with significant activated coagulation and inflammatory cascades and required high-dose anticoagulation with heparin. It may cause damages to newly transplanted lung and other organs such as the brain and kidney, and it may also cause bleeding and increase transfusion requirement.
Recently, extracorporeal membrane oxygenator (ECMO) is used as alternative modality of CPB in intraoperative cardiopulmonary support. ECMO has several advantages compared with CPB. ECMO is associated with lower heparin dose and reduced blood-activating surface due to lack of a venous reservoir and additional suction lines (3), thus attenuating coagulopathy and inflammatory cascade related to CPB. Furthermore, ECMO could be easily extended to postoperative care in case of postoperative graft dysfunction (3). Recently, some centers in Europe and North America changed their primary modality of intraoperative cardiopulmonary support from CPB to ECMO and reported the potential advantages of ECMO (3-7). These reports showed that improved short-term outcomes such as less bleeding and reoperation, less perioperative requirement for transfusion, fewer respiratory and renal complications, shorter mechanical ventilation (MV) duration, shorter intensive care unit (ICU) and hospital stay, and better short-term survival compared with CPB. All these centers were high-volume centers and ECMO or CPB was used on a selective basis.
Until February 2013, we used the CPB on the selective basis as the primary modality of intraoperative cardiopulmonary support. However, since March 2013, we routinely used ECMO in patients who underwent LTx. This retrospective study was conducted to evaluate the safety and advantages of the routine use of ECMO during LTx compared with the selective use of CPB.
Methods
Patients
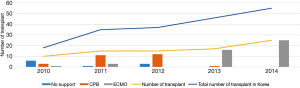
Between January 2010 and December 2014, 83 consecutive patients underwent LTx in our institution. After exclusion of 1 patient who underwent heart-LTx, 82 patients were included in this study. Between January 2010 and February 2013, 41 patients underwent LTx (group A), and 10 (24.4%), 27 (65.9%), and 4 (9.8%) patients underwent LTx under no support, under CPB support, and under ECMO support, respectively. During this period, CPB was the primary modality of cardiopulmonary support and was used on a selective basis. CPB was instituted in cases of hemodynamic instability or inability of the patient to tolerate pulmonary artery clamping or single-lung ventilation. Patients with preoperative ECMO or interventional lung assistance (iLA) underwent operation with ECMO support, and if the patient was not tolerable with ECMO support, CPB was instituted. Between March 2013 and December 2014, 41 patients underwent LTx (group B), and all these patients underwent LTx under venoarterial (VA) ECMO support (Figure 1). During this period, VA ECMO was instituted during anesthesia induction or after open-chest or hilar dissection. We retrospectively reviewed the data from our lung transplant database and the patients’ medical record. This study was approved by the Institutional Review Board, and the informed consent requirement was waived (IRB number 4-2015-1213).
LTx procedure
All organs were recovered en bloc from mechanically assisted brain-dead donors. A low-potassium dextran solution (Perfadex®; Duraent Biologicals, Hyderabad, India) was used as a preservation solution in all cases. Anterograde and retrograde flushing were used. Clamshell incision of the fourth intercostal space has been the preferred surgical approach in double LTx. Anterolateral thoracotomy was used in 1patient in each group who underwent single LTx. Double LTx was performed sequentially. Right side was usually implanted first. All patients received standard triple immunosuppression with calcineurin inhibitor (cyclosporine or tacrolimus), mycophenolate mofetil, and methylprednisolone.
CPB and extracorporeal membrane oxygenation (ECMO)
The right atrium (RA) and ascending aorta (AO) were used as cannulation sites in case of CPB except in one patient. Heparin (300 IU/kg) was administered before cannulation, and the target activated clotting time (ACT) was longer than 400 seconds. Protamine was given to antagonize heparin on weaning from CPB. The CPB system consisted of Stocker S5 (Sorin Group, Munich, Germany) heart-lung machine and roller pump and Capiox Rx25 oxygenator (Terumo, Tokyo, Japan). Cardiotomy suction was used while the recipient bronchus was closed. The suctioned blood was discarded as long as the recipient bronchus was open.
In the case of venovenous (VV) ECMO, the femoral and internal jugular veins (IJV) were the preferred cannulation sites, and usually, cannulation was done with percutaneous Seldinger technique. In the case of VA ECMO, the femoral vein (FV) and artery were the preferred cannulation sites. The FV and artery were exposed and prepared through inguinal incision and then cannulation using the Seldinger technique. When femoral vessels were unsuitable or insufficient for cannulation, either dual venous cannulation or central cannulation (RA or AO) was used. The right carotid artery (CA) was used in a patient with preoperative VA ECMO because of the patient’s small femoral artery (FA). Heparin (2,000 IU) was administered before cannulation, and the target ACT was 180−200 seconds during ECMO. Protamine was given only on weaning from ECMO intraoperatively. Bioline heparin-coated Quadrox PLS circuit system (Maquet Cardiopulmonary, Hirrlingen, Germany) or the Capiox emergency bypass system (Terumo) was used. A cell saver was used while the recipient bronchus was closed, and the suctioned blood was discarded as long as the recipient bronchus was open.
iLA (Novalung®; Oberstenfeld, Germany) was used in a patient preoperatively as a bridge to LTx, and the FA and vein were cannulated. This patient was supported with VA ECMO via previous femoral vessel cannulation during operation and included group A.
Data collection
Preoperative recipient variables included age, sex, body mass index (BMI), diagnosis, preoperative lung function, presence of pulmonary hypertension, preoperative MV, and preoperative extracorporeal life support (ECLS). Pulmonary hypertension was defined as a mean pulmonary arterial pressure >25 mmHg by right heart catheterization or a systolic pulmonary arterial pressure >40 mmHg by echocardiography (8,9). Donor variables included donor age, sex, MV time, partial pressure of oxygen (pO2) with 100% inspired O2, bronchoalveolar lavage (BAL) culture, and smoking history ≥20 pack-year.
Intraoperative variables included incision, intraoperative ECLS mode and cannulation site, double or single LTx, ischemic time, lung volume reduction due to size mismatch, intraoperative packed red blood cells (pRBC), platelet, fresh frozen plasma (FFP). Although the initial single-lung ventilation and clamping of the right pulmonary artery has not provoked any hemodynamic and respiratory instability with or without ECMO, CPB was instituted because of the instability during transplantation, and it was considered a non-elective CPB. Ischemic time was defined as the time between aortic cross-clamp in donation after brain-dead donors and reperfusion of the implanted lung.
Postoperative variables included prolonged and secondary ECMO use, complications, postoperative transfusion up to 48 hours, MV, ICU and hospital stay, survival, and postoperative lung function. ECMO use was considered prolonged if the ECMO was initiated in the operating room (OR) and the patient left the OR with a running ECMO system. If ECMO has to be re-inserted on the ICU after the patients left the OR, it was considered as secondary ECMO (4). Grade 3 primary graft dysfunction (PGD) was recorded at 24, 48 and 72 hours after LTx according to the guidelines of the International Society for Heart and Lung Transplantation (ISHLT) Working Group on PGD (10). Postoperative bleeding is defined as reoperation for bleeding or transfusion of more than 6 units of pRBC up to 48 hours after LTx (6). Rejection is defined as the need for at least 1 steroid pulse therapy in the presence of unexplained worsening of lung function and gas exchange and an increase in inflammatory parameters (3). Neurologic complication included cerebral infarction, intracranial hemorrhage, and/or seizure. Prolonged MV is defined as MV exceeding 21 days (11,12).
Statistical analysis
Categorical variables were compared using χ2 test or Fisher’s exact test. Continuous variables were compared using Students’ t-test or Mann-Whitney test. Survival was plotted with Kaplan-Meier curves and compared using the log-rank test. All tests were 2-sided, and P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Preoperative recipient and donor characteristics
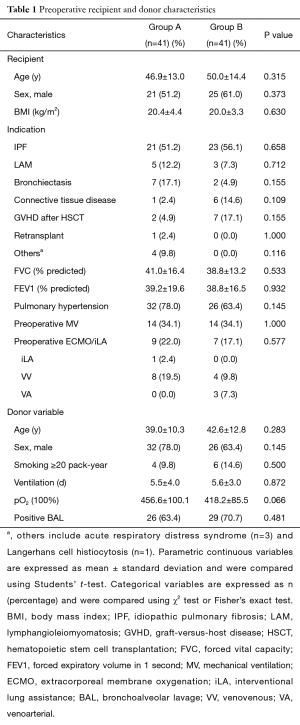
The characteristics of preoperative recipient and donor are summarized in Table 1. Idiopathic pulmonary fibrosis (IPF) was the major indication in both groups (group A, 21 patients; group B, 23 patients). The pO2 with 100% inspired O2 of donor in group A was higher than that in group B. However, it did not reach statistical significance (P=0.066), which might indicate that our donor selection criteria became somewhat less restrictive. Other variables did not differ between the two groups.
Full table
Intraoperative characteristics
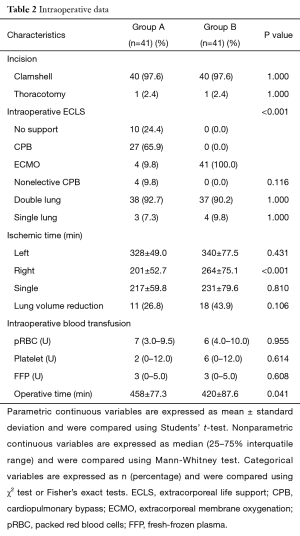
Intraoperative data are summarized in Table 2. Most patients underwent double LTx under clamshell incision. Four patients in group A were supported with non-elective CPB. All patients in group B were transplanted with VA ECMO, and none needed additional CPB support. The ischemic time of the right lung of patients in group B was significant longer than that of patients in group A (P<0.001). However, there was no significant difference in the ischemic time of the left lung. There was no significant difference in the transfusion amount of pRBC, platelet, or FFP. The operative time was significant longer in group A (group A, 458 min; group B, 420 min; P=0.041).
Full table
Early postoperative outcomes
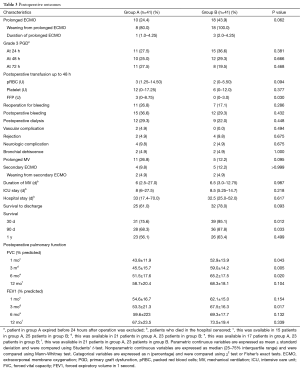
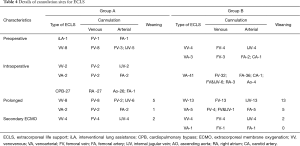
Postoperative outcomes are summarized in Table 3. More patients in group B were supported with prolonged ECMO postoperatively, but it did not reach significance (group A, 10 patients; group B, 18 patients; P=0.062). All patients supported with prolonged ECMO in group B were successfully weaned. There was no difference in PGD at 24, 48 and 72 hours after LTx. Patients in group B had less FFP transfusion (P=0.030). Packed RBC and platelet transfusion up to 48 hours after LTx, reoperation for bleeding and postoperative bleeding were not different between the two groups. Other measured complication profiles were not different between the two groups. There were two vascular complications in group A: one was compartment syndrome in a patient supported with intraoperative VA ECMO, and the other was aortic dissection in patient supported with intraoperative CPB. The details of cannulation site are summarized in Table 4. The duration of MV and ICU and hospital stay were not different between the two groups.
Full table
Full table
Survival and pulmonary functions after LTx
The patients in group B had statistically higher 30- and 90-day survival (P=0.012 and P=0.033, respectively). More patients survived to discharge; however, this did not reach statistical significance (group A, 61.0%; group B, 78.0%; P=0.093). The 1-year survival showed better trends in group B, but it was not significant (group A, 56.1%; group B, 63.4%, P=0.311) (Figure 2A). Comparison of the Kaplan-Meier survival curve by modality of intraoperative circulatory support showed that patients supported with ECMO showed better survival than patients supported with CPB (P=0.042) and a similar survival rate with patients with no support (P=0.380) (Figure 2B).

Patients in group B showed better pulmonary function than patients in group A. Forced vital capacity (FVC) at 1, 3, and 6 months after LTx was better in group B than that in group A (P=0.043, P=0.005, and P=0.020). Forced expiratory volume in 1 second (FEV1) at 3 months after LTx was better group B than that in group A (P=0.017) (Table 3).
Discussion
The LTx program in our institute started in July 1996 (13), which was first in Korea. Comparing the number of the total LTx in Korea from the Korean Network for Organ Sharing (KONOS, www.konos.go.kr), nearly half of the LTx in Korea has being conducted in our institute. Until December 2014, 111 LTx had been performed consecutively in our institute. Although our institute is the largest LTx center in Korea, we have limited annual procedure volume and experience, compared with higher-volume centers in Europe and North America (14).
Until February 2013, our primary modality for cardiopulmonary support was CPB, and it is used on a selective basis as with other high-volume centers in North America and Europe, where fewer than 50% of patients need extracorporeal support during LTx (3-7). However, only ten patients (24.4%) underwent LTx without extracorporeal support in our institute. Preoperative and postoperative use of ECMO is increasing, as 22% of patients used ECMO preoperatively as bridge to transplantation and 24% patient needed prolonged ECMO support postoperatively. Some investigators reported on improved clinical outcomes of LTx with ECMO (3,4), and thus we decided to change our strategy about intraoperative extracorporeal support. We applied VA ECMO on all patients who underwent LTx during operation.
After the routine use of VA ECMO during LTx, the operative time was shortened. Thus, despite the longer ischemic time of the right lung in group B than that of group A, the ischemic time of the left lung was not different. None of the patients needed additional or non-elective CPB. The requirement of transfusion during operation was not different, with less transfusion of FFP after operation. It can be attributed to the use of VA ECMO, which has several advantages. First, VA ECMO can maintain proper oxygenation and achieve hemodynamic support despite single-lung ventilation and manipulation of heart during LTx, and this is less dependent on anesthesiologist experience in LTx. It allows a better surgical field and decreases operative time and technical failure. This may give greater advantage to less experienced and low-volume centers like our institute. Second, VA ECMO needs less anticoagulation and has less priming volume. Thus, it does not increase the need for transfusion.
Complications related with extracorporeal circulation such as vascular and neurologic complications were not increased in group B. Our favored cannulation site in VA cannulation is the femoral vessel. It may decrease the risk of cerebral embolism compared with aortic and CA cannulation. One patient who underwent CA cannulation had severe cerebral embolism and died. Aigner et al. (4) had concerns about the increased risk of vascular complication when using the femoro-femoral route. To decreased vascular complications, we exposed and prepared femoral vessels first, and then performed cannulations. In case that the FA was not suitable for cannulation and there was greater risk of peripheral ischemia, we used another vessel such as AO or CA. No such vascular complication was present in group B.
In this present study, patients in group B had more improved postoperative pulmonary function. It may be caused by following; first, a significant volume in the RA bypassed through VA ECMO. Thus, VA ECMO may prevent overperfusion to the newly transplanted lung, decreased transplanted lung injury, and improved postoperative lung function (1). Second, more patients in group B are supported with prolonged ECMO postoperatively. Prolonged ECMO could allow the patients to be treated with lung protective ventilation with a decreased risk of hypoxia. The increased prolonged ECMO use could be the advantage of routine VA ECMO by peripheral cannulation. This can be easily extended to postoperative care without the risk of additional cannulation and hemodilution due to new priming.
The 30- and 90-day survival rates were improved with routine ECMO strategy. Furthermore, in the comparison of survival by intraoperative extracorporeal support, ECMO patients had significantly improved survival compared with CPB patients, and had no difference with patients who underwent LTx without support.
Most LTx were performed in high-volume centers in Europe and North America. According to the data from the registry of ISHLT in 2015, 14 centers (9%) with ≥50 LTx annually performed 33% of the total procedures and 13 (93%) of these 14 centers were in Europe and North America. Outside Europe and North America, 20 centers reported their data to ISHLT, and among them, only 1 (5%) center had ≥50 cases (14). Outside Europe and North America, the number of LTx may not be enough and the majority of existing LTx centers may have less experience and lower center volume. These centers may suffer from poor outcomes (15,16).
Some high-volume centers in Europe and North America reported the advantage of ECMO compared with CPB (3-7). However, they use ECMO on a selective basis and more than 50% of patients were performed LTx without cardiopulmonary support with excellent outcomes (17,18). However, our institute has limited experience and lower annual volume. Moreover, our patients had more risk factors such as IPF, preoperative pulmonary hypertension, preoperative MV, and preoperative ECMO/iLA. These characteristics might be attributed to late referral of patients to a transplantation center by a clinician who is not familiar with LTx or the urgency-based organ-allocation system in Korea. In such circumstances, ECMO may be more advantageous and can result to improved short-term outcomes. Our experience could be a lesson and be applied to other newly established centers.
Our study has several limitations. First, this was a retrospective, single-center study with small sample size. However, as already mentioned, there are a few LTx centers outside Europe and North America, which also have limited experience and low LTx volumes. LTx volume is related with outcomes (15,16). The advantage of ECMO and the results during LTx could be different in these less experience and lower volume center, and this is rarely reported. Second, there may be temporal bias and higher annual volume in group B. However, there was no significant change in postoperative care and operative technique. With routine use of ECMO during operation and its extension to postoperative care, we could extend donor criteria. Thus, increased annual LTx volume could be the result of routine ECMO.
Conclusions
In conclusion, routine use of ECMO during LTx could improve short-term survival and postoperative lung function without increased extracorporeal-related complication such as vascular and neurologic complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board, and the informed consent requirement was waived (IRB number 4-2015-1213).
References
- Marczin N, Royston D, Yacoub M. Pro: lung transplantation should be routinely performed with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2000;14:739-45. [Crossref] [PubMed]
- McRae K. Con: lung transplantation should not be routinely performed with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2000;14:746-50. [Crossref] [PubMed]
- Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012;144:1510-6. [Crossref] [PubMed]
- Aigner C, Wisser W, Taghavi S, et al. Institutional experience with extracorporeal membrane oxygenation in lung transplantation. Eur J Cardiothorac Surg 2007;31:468-73; discussion 473-4. [Crossref] [PubMed]
- Bermudez CA, Shiose A, Esper SA, et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann Thorac Surg 2014;98:1936-42; discussion 1942-3.
- Biscotti M, Yang J, Sonett J, et al. Comparison of extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2014;148:2410-5. [Crossref] [PubMed]
- Machuca TN, Collaud S, Mercier O, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2015;149:1152-7. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46:903-75. [Crossref] [PubMed]
- Jeon CH, Chai JY, Seo YI, et al. Pulmonary hypertension associated with rheumatic diseases: baseline characteristics from the Korean registry. Int J Rheum Dis 2012;15:e80-9. [Crossref] [PubMed]
- Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2005;24:1454-9. [Crossref] [PubMed]
- Hadem J, Gottlieb J, Seifert D, et al. Prolonged Mechanical Ventilation After Lung Transplantation-A Single-Center Study. Am J Transplant 2016;16:1579-87. [Crossref] [PubMed]
- MacIntyre NR, Epstein SK, Carson S, et al. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest 2005;128:3937-54. [Crossref] [PubMed]
- Haam SJ, Lee DY, Paik HC. An overview of lung transplantation in Korea. Transplant Proc 2008;40:2620-2. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1264-77. [Crossref] [PubMed]
- Weiss ES, Allen JG, Meguid RA, et al. The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg 2009;88:1062-70. [Crossref] [PubMed]
- Thabut G, Christie JD, Kremers WK, et al. Survival differences following lung transplantation among US transplant centers. JAMA 2010;304:53-60. [Crossref] [PubMed]
- Bittner HB, Binner C, Lehmann S, et al. Replacing cardiopulmonary bypass with extracorporeal membrane oxygenation in lung transplantation operations. Eur J Cardiothorac Surg 2007;31:462-7; discussion 467. [Crossref] [PubMed]
- Ius F, Sommer W, Tudorache I, et al. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: Indications and midterm results. J Heart Lung Transplant 2016;35:49-58. [Crossref] [PubMed]


