Role of plasma MicroRNAs in the early diagnosis of non-small-cell lung cancers: a case-control study
Introduction
Lung cancer remains one of the leading causes of cancer mortality not only in the worldwide but also in China. In the past 50 years, the mortality and incidence rate have been rising in many countries. On average, about 600,000 cases were reported to die of lung cancer in China every year (1). About 80% of lung cancers were non-small-cell lung cancers (NSCLC), including three main subtypes: squamous cell carcinoma (SQ), adenocarcinoma (AC), and large-cell carcinoma. Although surgery, chemotherapy, radiotherapy and target therapy have significantly improved the prognosis, the 5-year survival rate for NSCLC remains at about 10% (2-4). Most patients are diagnosed at a late stage and lose the best chance to treat, and the majority of them can only be treated with palliative therapy. This causes the overall survival remaining poor, and many patients die within a few months of diagnosis. Therefore, it is very important to improve the survival rate for NSCLC by early stage diagnosis and treatment.
Currently, the reliable methods to diagnose NSCLC were developed, such as computed tomography (CT), magnetic resonance imaging (MRI) and biopsy; however, it was still hard to give an early diagnosis of the disease in clinical practice. Most patients are often diagnosed in the middle-late disease period. Biopsy with histopathological examination is usually used to confirm the diagnosis. But its clinical application is limited because of the difficulty in obtaining biopsy tissue and the trauma to patients. Thus novel biomarkers used for early diagnosis, which are easier to be obtained and tested, are urgently needed. Novel markers from plasma, such as CEA, SCCA, NSE, CRP, CYFRA21-1, and microRNAs (miRNAs) used for early diagnosis of NSCLC, are attracting intense interest recently, which are more convenient, with lower cost and smaller trauma than traditional methods (5-7).
MiRNAs are a group of small non-coding, single-stranded RNAs, acting as negative regulators of gene expression at the post-transcriptional level. Over 2,500 miRNAs are transcribed from miRNA genes in the human genome (8). There is a variety of biological functions of miRNAs, such as differentiation, proliferation, cellular development, cell death and metabolism (9). Cancer initiation and progression can involve miRNA, and past studies have indicated that miRNAs play an important role in regulating those biological functions, including apoptosis, which is a process frequently evaded in cancer progression. Their expression profiles can be used for the classification, diagnosis, and prognosis of human malignancies. In the last decade, miRNAs measured either from tumor samples or in biofluids, have emerged as biomarkers for tumor diagnosis, prognosis and prediction of response to treatment. MiRNAs provide the possible approach to earlier diagnosis of lung cancer. Deregulation happens among some miRNAs in lung cancer, which target cancer-relevant events and have been evidenced to have tumor-suppressing or tumor-promoting activity both in vitro and in vivo models in lung cancer (10). CYFRA21-1 is a small part of cytokeratin (CK) 19, which is the main structural element of the cytoskeleton of epithelial cells. CK19 has been reported to be over-expressed in many lung cancer tissue specimens (11,12), which results in an increase of the plasma CYFRA21-1 values (13). Some studies reported that CYFRA21-1 can be useful for pathological typing and assessment of treatment efficacy of NSCLC (14,15).
However, there are also opposite views about the miRNAs being a biological marker for lung cancer diagnosis and prognosis, and the sensitivity and specificity of the miRNAs in early lung cancer diagnosis, which reflect that the lung cancer progression is still not very clear. Some studies reported that plasma tumor markers with high concentrations are often found only when the disease is at an late stage (16-19). Therefore, it is difficult to detect a lung tumor clinically at an early stage with plasma marker assays (20,21). In addition, it is also unknown that whether there is an optimal cut-off value to discriminate patients from non-cancer people. In this study, the role of miRNAs and other plasma markers were evaluated in the diagnosis of 59 NSCLC patients.
Methods
Study population
A 1:1 matching case-control study was conducted in Department of Thoracic Surgery of Xuanwu Hospital from January 2012 to December 2014 in Beijing, China. This study was approved by the Ethics Committee of Xuanwu Hospital (ID: clinical research 2014022) and written informed consent was obtained from all study participants. Patients who were confirmed at an early stage of NSCLC (I–IIIA) from pathological or cytological perspectives recommended by WHO were recruited to participate in the study. The inclusion criteria were as follows: confirmed diagnosis of lung cancer for the first time, at the stage I–IIIA, age of 18 or more, had willingness to participate in the study, and had not been previously diagnosed with other cancers. Tumors were staged according to the tumor-node-metastasis (TNM) staging system of the American Joint Committee on Cancer.
Control group
The controls were recruited from among patients confirmed lung benign disease by the department of lung disease in the same Hospital and during the same study period. The controls had no history of lung cancer or other cancers. They were matched with the cases by the same sex and similar age (being in ±2 years).
Experimental data collection
All study participants underwent a routine medical examination. Their age, sex, disease history, and laboratory data were all recorded. A 10 mL sample of venous blood was collected in an anticoagulant tube with ethylene diamine tetra acetic acid (EDTA) among all study participants before any therapies of the patients or before the patients started treatment or underwent lung resections. Then the blood samples were centrifuged at 1,000 g for 10 min at 4 °C in a Sigma 3k15 centrifuge (SIGMA Laborzentrifugen, Osterode am Harz, Germany) and the plasma was immediately separated, frozen and stored at −80 °C until analysis. MiRNAs, SCCA, CEA, and CYFRA21-1 were tested from all the subjects.
Relative changes of ten miRNAs (miR-)expression including miR-126, miR-150, miR-155, miR-205, miR-21, miR-210, miR-26b, miR-34a, miR-451 and miR-486 were selected to analyze between the case and control group. These ten miRNAs shows altered expressions in lung cancer plasma in previous studies. The experimental steps were as follows: firstly, the total RNA were extracted and purified from 200 µL of plasma using the QIAGEN MiRNeasy Mini Kit (catalogue no. 217004; QIAGEN, Hilden, Germany). The quantity and quality of RNA were measured by a dual-beam ultraviolet spectrophotometer (Beckman Coulter, Fullerton, CA, USA) and samples with a A260/A280 ratio 1.8:2.1 were used for analysis. Secondly, the plasma concentration of ten miRNAs was measured by quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) using the QuantiTectTM SYBR Green PCR Kit (QIAGEN) and the Applied Biosystems 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) according to the manufacturers’ instructions. MiRNA specific stem-loop primers were supplied by the TaqMan MiRNA Assays (Applied Biosystems, Inc., Grand Island, NY, USA) and the reverse transcription reactions from miRNAs converting to cDNA were conducted with TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Inc., Grand Island, NY, USA). The reactions were incubated in a 96-well optical plate at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, and 60 °C for 60 s. Reactions were performed in triplicate. Thirdly, the cycle threshold (CT) was recorded, which was defined as the number of PCR cycles required for the fluorescent signal to be higher than a threshold indicating baseline variability. miRNA-16 (sequence: 0-UAGCAGCACGUAAAUAUUGGCG-30; Takara Biotechnology) was chosen as the endogenous reference control. Relative changes of miRNA expression were represented by 2-ΔCT, and the differences between the original copy number of miRNA in the lung cancer group and that in the control group were analyzed, where
Other tumor markers were also assayed according to the protocol. CYFRA21-1 levels were measured by an electrochemiluminescent immunoassay (ECLIA) (CYFRA21-1; Roche Diagnostics, Germany). CEA levels were measured by a chemiluminescence immunoassay (CLIA) (Centaur CEA; Bayer HealthCare, USA). SCC levels were measured by an immunoradiometric assay (IRMA) (SCC-RIABEAD; SRL Inc., Japan).
Statistical analysis
Statistical analysis is performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) for Windows. Categorical data are expressed as frequencies. Pearson’s Chi-square test or Fisher’s exact test is used to analyze the differences of categorical variables. Continuous data are expressed as the mean ± standard deviation. Student’s t-test or the Mann-Whitney U-test is used to analyze the differences of continuous variables between groups. Receiver-operating-characteristic (ROC) analysis is used to analyze the ability of the plasma markers level to discriminate between patients and controls. The areas under the ROC curves (AUROCs) are calculated to assess the performance of each marker to distinguish lung cancer. Optimum cut-off values are calculated to optimize sensitivity and specificity (i.e., the Youden index). The positive and negative predictive values [the positive predictive value (PPV) and the negative predictive value (NPV), respectively] of each marker and combined markers are assessed. When analyzing the diagnostic yield of a combination of tumor markers, a case is considered as positive if either tumor marker result is positive, and negative if both or three tumor marker results are negative. A P<0.05 (two-tailed) is considered as the level of statistically significant.
Results
Baseline clinical characteristics of the study population
A total of 59 NSCLC patients and controls, including 41 males and 18 females respectively, were selected in the study. Among the 59 patients, 35 were at stage I–II and 24 were at stage IIIa. According to the WHO classifications, 30 patients (50.8%) were diagnosed with SQ, 26 (44.1%) with AC, 3 (5.1%) had large-cell carcinoma. The 59 controls that were diagnosed with lung benign diseases included 32 cases of pulmonary infection, 17 cases of chronic obstructive pulmonary diseases and 10 cases of pulmonary tuberculosis. The average age of the case group was 55.9±15.1 years and the control group was 57.6±16.5 years. The independent t analysis showed that there were no significant differences between the two groups’ age (t=0.58, P=0.72) (See Table 1).
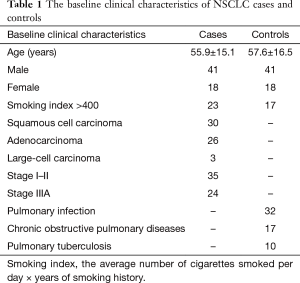
Full table
Comparison of plasma CEA, CYFRA21-1, SCC and miRNAs
Plasma CEA, SCC, CYFRA21-1 and ten candidate miRNAs levels were observed and analyzed. CYFRA21-1, miR-486 and 210 levels were significantly different in patients with NSCLC than those in controls. CEA, SCC, and other miRNAs were not significantly different in two groups (See Table 2).
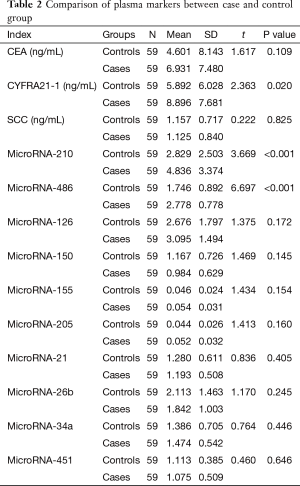
Full table
ROC curve analysis
AUROC of CYFRA21-1, miR-486 and miR-210 were 0.624 (sensitivity: 0.576; specificity: 0.797), 0.848 (sensitivity: 0.831; specificity: 0.780) and 0.751 (sensitivity: 0.746; specificity: 0.746), respectively. The optimal cut-off value of CYFRA21-1, miR-486 and miR-210 were 6.595, 1.988 and 3.341, respectively to discriminate patients from controls (See Figure 1 and Table 3). MiR-486, miR-210 and CYFRA21-1 combined diagnosis ability was the highest, and the AUC was 0.924 (sensitivity: 0.847; specificity: 0.728) (See Table 4, Figure 1). There were two cut-off values of miR-210 from the ROC. When the cut-off value of miR-210 was 3.398, the PPV and NPV were both lower than those of 3.341 (0.729 vs. 0.746 for both), so 3.341 was selected as the cut-off value.
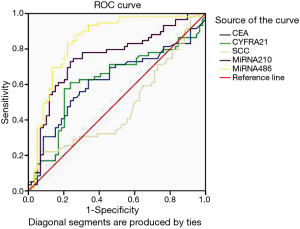
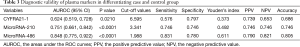
Full table

Full table
Discussion
When tumor metastasis occurs, it often results in treatment failure of advanced lung cancer. So early diagnosis of lung cancer is essential for prognosis of the patients (22). But lack of diagnostic biomarkers for early detection has made lung cancer one of the human cancers with the worst prognosis. In clinical practice, lung cancer diagnosis still depends primarily on imaging techniques, such as X-ray and CT, and if a suspicious lesion is present, biopsy with histopathological examination is used to confirm the diagnosis. The early detection of metastasis in lung cancer patients using a convenient method becomes an important research goal (23). This study we found that tumor marker levels were higher in NSCLC patients than those patients with benign lung disease (Table 2). To evaluate the diagnostic value of tumor markers, we used ROC curves to calculate cut-off levels and AUC. We observed a moderate specificity (86.7%) and high sensitivity (91.3%) when combined cut-off levels of tumor markers were used. This study was designed to evaluate the diagnosis value of tumor markers miRNAs, CYFRA21-1, CEA, and SCC in differential diagnosis of early-stage NSCLC from benign lung disease. It showed that plasma CYFRA21-1, miR-486 and 210 were different between NSCLC and controls, but CEA and SCC had no differences. MiR-210 and miR-486 had higher sensitivity and accuracy, but those of CYFRA21-1 was lower in the early diagnosis for NSCLC.
Plasma CYFRA21-1 level has been reported for diagnosis of SQ of lung cancer with high specificity (24). This study also got the high diagnosis specificity of early stage lung cancer but low sensitivity. But there were slightly different conclusions about CYFRA21-1 being a sensitive tumor marker for lung cancer. A study (25) had confirmed that plasma CYFRA21-1 appeared more sensitive and more specific for lung cancer diagnosis than other tumor markers such as CEA and SCC. A meta-analysis has also revealed that plasma CYFRA21-1 may be a biomarker in the diagnosis of patients with NSCLC (26). Our study also showed that combined with other plasma markers, CYFRA21-1 had a higher sensitivity.
In this study, miR-486 was demonstrated to be higher in plasma of early-stage NSCLC than that in controls, which was a sensitive and specific marker in plasma for differentiating of patients from “normal” individuals. Compared to other markers in this study, miR-486 showed the best ability to independently screen early-stage NSCLC according to diagnostic validity assessing indexes such as AUROC, Youden’s index, PPV, NPV and Accuracy. So the researchers believed that miR-486 could become a promising marker in peripheral blood for early detection of NSCLC. MiR-486 had been reported to be a significant biomarker for clinical assessment of lung cancer patients in many studies. But different studies reported different conclusions about miR-486’s regulation in cancers. Some studies reported that miR-486 was reduced in peripheral blood or tissues (27-29) of lung cancer patients, while others reported that it was up-regulation in lung cancer patients (30) or those patients with up-regulated level miR-486 in plasma had short overall survival (31). Those inconsistent findings were controversial and different study samples selection may be an important reason for this, which needs to be further studied and comprehensively analyzed.
In this study, miR-210 was another plasma miRNA marker for early detecting NSCLC. The plasma level of miR-210 in patients with early stage was significantly higher than that in controls. According to the AUROC, Youden’s index, PPV, NPV and accuracy, miR-210 was the second most significant plasma marker for early diagnosis compared to other markers in this study. At present, Among miRNAs studies, miR-210 has become one of the most widely studied one in cancer, which was reported to be upregulated in various types of cancers (32,33) and has played an important role in tumorigenesis. This result was consistent with previous findings in which miR-210 levels were increased in lung cancer patients (34-37). MiR-210 levels expression also was a prognostic factor for survival of lung cancer patients (38). However, the mechanism of miR-210 upregulation has not been completely clear. MiR-210 overexpression showed a correlation with hypoxia-inducible factor, carbonic anhydrase IX, and with von Hippel-Lindau mutation or promoter methylation (39). Although it is still not clear enough in the exact mechanism of miR-210 action and its role in tumorigenesis, it is of great significance in exploring its application as a diagnostic method in many types of cancers. It indicates that plasma miR-210 may play a role as a novel diagnostic marker of early stage lung cancer.
Circulating miRNAs have been reported to be remarkable biomarkers for cancer diagnosis due to their abundance and stability in circulating blood (40). Our study identified two candidate miRNAs, those are miR-486 and miR-210, as potential blood-based biomarkers for early diagnosis and prognosis of NSCLC. Circulating tumor-derived miRNAs were first described in peripheral blood by Mitchell et al. (41), who found that circulating miRNAs had the potential to be new biomarkers in patients with prostate cancers. MiRNAs from blood also demonstrated high stability after prolonged incubation at room temperature and/or after multiple freezing-thawing processes. Thus, identifying a miRNA that can diagnose lung cancer patients, at an early stage, may play an important role in the future treatment of this cancer (42).
In this study, two of the three plasma markers or the three in parallel combined diagnosis ability were higher than single marker, particularly miR-486, miR-210 and CYFRA21-1 in parallel. However, this study has several limitations to be taken into account: firstly, this study was an hospital based case-control study, it was convenient and less cost, but the samples were selected from the same department of the same hospital, it is necessary to consider the representativeness of the sample when the results are generalized. Secondly, the sample size in this study is relatively small, in which only 59 NSCLC patients and non-cancer controls were recruited, the results is possible to be validated in a significantly larger group of patients with NSCLC. Therefore, further large sample populations and multicentric studies are needed to confirm these findings. Thirdly, only parts of plasma markers were analyzed and the diagnostic value of plasma markers may be affected by some factors, such as prevalence rate of disease. Therefore, conclusions obtained from this study may be limited in other studies.
Acknowledgements
The authors thank the staffs at Xuanwu Hospital and Beijing Chao-Yang Hospital who did much work on the study.
Funding: This work was supported by the project “Research on Key Techniques of Early Detection and Standardized Therapy of Lung Cancer” of Beijing science and Technology Commission (D141100000214002)
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Xuanwu Hospital (ID: clinical research 2014022) and written informed consent was obtained from all study participants.
References
- Accessed Dec 3, 2015. Available online: http://www.chyxx.com/news/2014/0409/236571.html
- Yang J, Zhu J, Zhang YH, et al. Lung Cancer in a Rural Area of China: Rapid Rise in Incidence and Poor Improvement in Survival. Asian Pac J Cancer Prev 2015;16:7295-302. [Crossref] [PubMed]
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. [Crossref] [PubMed]
- Wink KC, Roelofs E, Solberg T, et al. Particle therapy for non-small cell lung tumors: where do we stand? A systematic review of the literature. Front Oncol 2014;4:292. [Crossref] [PubMed]
- Indovina P, Marcelli E, Maranta P, et al. Lung cancer proteomics: recent advances in biomarker discovery. Int J Proteomics 2011;2011:726869.
- Dietel M, Bubendorf L, Dingemans AM, et al. Diagnostic procedures for non-small-cell lung cancer (NSCLC): recommendations of the European Expert Group. Thorax 2016;71:177-84. [Crossref] [PubMed]
- I H, Cho JY. Lung Cancer Biomarkers. Adv Clin Chem 2015;72:107-70. [Crossref] [PubMed]
- Chaulk SG, Ebhardt HA, Fahlman RP. Correlations of microRNA:microRNA expression patterns reveal insights into microRNA clusters and global microRNA expression patterns. Mol Biosyst 2016;12:110-9. [Crossref] [PubMed]
- Del Vescovo V, Grasso M, Barbareschi M, et al. MicroRNAs as lung cancer biomarkers. World J Clin Oncol 2014;5:604-20. [Crossref] [PubMed]
- Joshi P, Middleton J, Jeon YJ, et al. MicroRNAs in lung cancer. World J Methodol 2014;4:59-72. [Crossref] [PubMed]
- Kosacka M, Jankowska R. Comparison of cytokeratin 19 expression in tumor tissue and serum CYFRA 21-1 levels in non-small cell lung cancer. Pol Arch Med Wewn 2009;119:33-7. [PubMed]
- Hanada S, Nishiyama N, Mizuguchi S, et al. Clinicopathological significance of combined analysis of cytokeratin19 expression and preoperative serum CYFRA21-1 levels in human lung squamous cell carcinoma. Osaka City Med J 2013;59:35-44. [PubMed]
- Dohmoto K, Hojo S, Fujita J, et al. The role of caspase 3 in producing cytokeratin 19 fragment (CYFRA21-1) in human lung cancer cell lines. Int J Cancer 2001;91:468-73. [Crossref] [PubMed]
- Wang B, He YJ, Tian YX, et al. Clinical utility of haptoglobin in combination with CEA, NSE and CYFRA21-1 for diagnosis of lung cancer. Asian Pac J Cancer Prev 2014;15:9611-4. [Crossref] [PubMed]
- Jung M, Kim SH, Lee YJ, et al. Prognostic and predictive value of CEA and CYFRA 21-1 levels in advanced non-small cell lung cancer patients treated with gefitinib or erlotinib. Exp Ther Med 2011;2:685-693. [PubMed]
- Shen L, Wan Z, Ma Y, et al. The clinical utility of microRNA-21 as novel biomarker for diagnosing human cancers. Tumour Biol 2015;36:1993-2005. [Crossref] [PubMed]
- Moldovan L, Batte KE, Trgovcich J, et al. Methodological challenges in utilizing miRNAs as circulating biomarkers. J Cell Mol Med 2014;18:371-90. [Crossref] [PubMed]
- Müller V, Gade S, Steinbach B, et al. Changes in serum levels of miR-21, miR-210, and miR-373 in HER2-positive breast cancer patients undergoing neoadjuvant therapy: a translational research project within the Geparquinto trial. Breast Cancer Res Treat 2014;147:61-8. [Crossref] [PubMed]
- Yang JS, Li BJ, Lu HW, et al. Serum miR-152, miR-148a, miR-148b, and miR-21 as novel biomarkers in non-small cell lung cancer screening. Tumour Biol 2015;36:3035-42. [Crossref] [PubMed]
- Seemann MD, Beinert T, Fürst H, et al. An evaluation of the tumour markers, carcinoembryonic antigen (CEA), cytokeratin marker (CYFRA 21-1) and neuron-specific enolase (NSE) in the differentiation of malignant from benign solitary pulmonary lesions. Lung Cancer 1999;26:149-55. [Crossref] [PubMed]
- Niklinski J, Furman M, Chyczewska E, et al. Diagnostic and prognostic value of the new tumour marker CYFRA 21-1 in patients with squamous cell lung cancer. Eur Respir J 1995;8:291-4. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Sharma D, Newman TG, Aronow WS. Lung cancer screening: history, current perspectives, and future directions. Arch Med Sci 2015;11:1033-43. [PubMed]
- Pujol JL, Grenier J, Daurès JP, et al. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21-1 immunoradiometric assay as a marker of lung cancer. Cancer Res 1993;53:61-6. [PubMed]
- Wieskopf B, Demangeat C, Purohit A, et al. Cyfra 21-1 as a biologic marker of non-small cell lung cancer. Evaluation of sensitivity, specificity, and prognostic role. Chest 1995;108:163-9. [Crossref] [PubMed]
- Cui C, Sun X, Zhang J, et al. The value of serum Cyfra21-1 as a biomarker in the diagnosis of patients with non-small cell lung cancer: a meta-analysis. J Cancer Res Ther 2014;10 Suppl:C131-4. [Crossref] [PubMed]
- Solomides CC, Evans BJ, Navenot JM, et al. MicroRNA profiling in lung cancer reveals new molecular markers for diagnosis. Acta Cytol 2012;56:645-54. [Crossref] [PubMed]
- Peng Y, Dai Y, Hitchcock C, et al. Insulin growth factor signaling is regulated by microRNA-486, an underexpressed microRNA in lung cancer. Proc Natl Acad Sci U S A 2013;110:15043-8. [Crossref] [PubMed]
- Wang J, Tian X, Han R, et al. Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer. Oncogene 2014;33:1181-9. [Crossref] [PubMed]
- Li W, Wang Y, Zhang Q, et al. MicroRNA-486 as a Biomarker for Early Diagnosis and Recurrence of Non-Small Cell Lung Cancer. PLoS One 2015;10:e0134220. [Crossref] [PubMed]
- Hu Z, Chen X, Zhao Y, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol 2010;28:1721-6. [Crossref] [PubMed]
- Fedorko M, Stanik M, Iliev R, et al. Combination of MiR-378 and MiR-210 Serum Levels Enables Sensitive Detection of Renal Cell Carcinoma. Int J Mol Sci 2015;16:23382-9. [Crossref] [PubMed]
- Haldrup C, Kosaka N, Ochiya T, et al. Profiling of circulating microRNAs for prostate cancer biomarker discovery. Drug Deliv Transl Res 2014;4:19-30. [Crossref] [PubMed]
- Eilertsen M, Andersen S, Al-Saad S, et al. Positive prognostic impact of miR-210 in non-small cell lung cancer. Lung Cancer 2014;83:272-8. [Crossref] [PubMed]
- Puisségur MP, Mazure NM, Bertero T, et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ 2011;18:465-78. [Crossref] [PubMed]
- Miko E, Czimmerer Z, Csánky E, et al. Differentially expressed microRNAs in small cell lung cancer. Exp Lung Res 2009;35:646-64. [Crossref] [PubMed]
- Li ZH, Zhang H, Yang ZG, et al. Prognostic significance of serum microRNA-210 levels in nonsmall-cell lung cancer. J Int Med Res 2013;41:1437-44. [Crossref] [PubMed]
- Osugi J, Kimura Y, Owada Y, et al. Prognostic Impact of Hypoxia-Inducible miRNA-210 in Patients with Lung Adenocarcinoma. J Oncol 2015;2015:316745.
- Neal CS, Michael MZ, Rawlings LH, et al. The VHL-dependent regulation of microRNAs in renal cancer. BMC Med 2010;8:64. [Crossref] [PubMed]
- He WJ, Li WH, Jiang B, et al. MicroRNAs level as an initial screening method for early-stage lung cancer: a bivariate diagnostic random-effects meta-analysis. Int J Clin Exp Med 2015;8:12317-26. [PubMed]
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008;105:10513-8. [Crossref] [PubMed]
- Barger JF, Nana-Sinkam SP. MicroRNA as tools and therapeutics in lung cancer. Respir Med 2015;109:803-12. [Crossref] [PubMed]


