Minimalist video-assisted thoracic surgery biopsy of mediastinal tumors
Introduction
Mediastinal tumors include a broad spectrum of malignancies thus calling for prompt diagnosis and early establishment of an appropriate treatment.
Video-assisted thoracic surgery (VATS) can be employed in this setting to obtain large tissue samples and/or whenever concomitant conditions requiring surgical management do develop. However, in patients with compression of airway and major vessels due to the presence of bulky masses, one should take into account the potential risk of general anesthesia and one-lung ventilation, which has been associated to life-threatening complications (1-5).
In recent years, there is an increasing interest towards non-intubated thoracic surgery strategies, which associate VATS to less invasive anesthesia protocols including locoregional anesthesia with maintenance of spontaneous breathing. In addition, other methodological and technological advances including uniportal surgical approaches and the use of small-sized instrumentation are being adopted in order to minimize the overall invasiveness of VATS thus defining novel minimalist surgical strategies (MVATS) (6-13).
Taking into account this concept and on the basis of previous findings achieved by MVATS in other delicate patients subgroups (6-8), we have explored the applicability of this ultra-minimally invasive surgical strategy in patients with undetermined mediastinal tumors in order to assess feasibility, safety and results.
Methods
Study design
This retrospective study is a part of a broader investigational program of MVATS on approval of the Institutional Review Board of Tor Vergata University Hospital. Indication for surgical biopsy was established in the context of a multidisciplinary meeting and all patients signed a consent form after detailed discussion on expected benefits and drawbacks of the scheduled surgical plan.
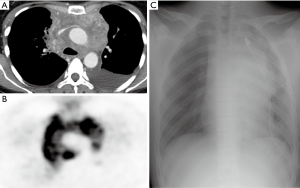
Chest computed tomography (CT) was carried out in all patients whereas positron emission tomography was carried out in non-urgent management setting as appropriate (Figure 1).
Primary outcome measures were technical feasibility scored into four grades (Table 1), operative morbidity defined as occurrence of any perioperative adverse event according to the Clavien-Dindo classification. Secondary outcome measures included diagnostic yield, global surgical time (total time spent in the operating theatre), and hospital stay. Results were also compared both by overall and propensity score matched analysis, to those reported in a group of patients who received VATS biopsy under general anesthesia and single-lung mechanical ventilation.
Statistical analysis
For propensity-score matching, the predicted probability (PP) of being treated by MVATS was calculated for each patient according to a logistic regression analysis. To this purpose, factors possibly affecting the decision on anesthesiology regimen were included in the multivariate model. These included age, performance status and mediastinal-thoracic ratio (MTR) (i.e., the ratio between the diameter of the mass and the width of the thorax at T5–T6 axial level on CT scan). The latter factor was dichotomized using a value of 0.35 as the cutoff, based on previous literature (3). A K nearest-neighbour algorithm was rank in order to match patients from both groups who had similar PPs. Group descriptive statistics are presented as median with quartile range (IQR). Due to the limited sample size and the non-normal distribution of data, the non-parametric Mann-Whiney U-test was used for comparison of unpaired data. Frequencies were assessed with a two-tailed Fischer’s exact test. A P value <0.05 was set as the significance threshold. The SPSS package (version 21.0) was used for all the statistical tests.
Anesthesia and surgical technique
In MVATS anesthetic management entailed maintenance of spontaneous ventilation and either a thoracic epidural catheter inserted at the T4–T5 level (6-10) or intercostal injection of lidocaine 2% plus ropivacaine 7.5% at the site(s) of surgical incision for thoracic analgesia. Physiologic patient monitoring included radial artery catheterization, bispectral index monitoring, and a capnograph stuck to a nostril for real-time end-tidal CO2 assessment. Equipment for emergent conversion to general anesthesia included a videolaryngoscope and a chest drainage unit ready for use on the sterile back table. Perioperative sedation was usually avoided in order to preserve patient’s ability to obey commands.
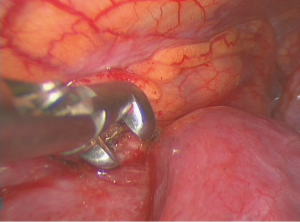
The patient was placed in a lateral or semilateral (30-degree) decubitus position with mild trunk elevation. One 20-mm wound protector (Mini-Alexis, Tyco Healthcare) was inserted between the 4th and 7th intercostal space, depending on the anatomic location of the tumor. A 30-degree, 10 mm camera was employed to facilitate oblique vision of the anterior mediastinum during spontaneous ventilation. Multiple biopsies were then taken with 5-mm sized spoon-shaped forceps (Figure 2). Additional measures such as talc pleurodesis, pleural biopsy, pericardial fenestration and wedge resection of pulmonary lesions were additionally performed whenever required.
One chest tube was inserted at the end of the procedure. Before wound closure, the chest tube was connected to water seal while the patient was asked to cough repeatedly to achieve complete lung re-expansion.
Postoperative care
Postoperative care entailed brief stay in the recovery room and transfer to the ward as soon as vital parameters were considered satisfactorily stable. On arrival, intravenous fluid administration was discontinued, and oral intake was allowed within one hour. If epidural anesthesia was employed, the catheter was removed 24 h after the operation and oral analgesia was started. Criteria for discharge were standardized and included stable and satisfactory clinical condition and removal of the chest tube if 24 h drainage amount ≤200 mL and no air leak was confirmed by temporary (1 h) tube clamping/declamping maneuver.
Results
Baseline data
There were 16 males and 8 females, with a median age of 53.5 years (IQR, 37.75–56.75 years). Thoracic epidural anesthesia was employed in 14 patients (54.2%), whereas in the remaining 11 patients (45.8%) the operation was carried out using local anesthesia. Median performance status was 1 (IQR, 0.25–2.0) although 7 patients (29%) had a performance status of 2 or more. Eight patients had concomitant pleural effusion, 4 patients had pericardial effusion and 3 patients had radiological evidence of associated lung nodules or ground glass opacities. Four patients had a low oxygenation with a PaO2/FiO2 ratio <300. Three patients had previously received non-surgical biopsy with inconclusive (N=1) or incomplete (N=2) results requiring more tissue for immunohistochemistry assay.
Surgical results
Out of the 24 procedures, 18 (75%) involved the anterior mediastinum, while 6 procedures involved the subcarinal (N=4) or paratracheal region (N=2). There were 16 right and 8 left approaches.
Technical feasibility was scored as grade 4 (excellent), 3 (good) and 2 (satisfactory) in 16, 6 and 2 patients, respectively (Table 1). Conversion to general anesthesia was never required. Global operating room time was 67.5 min (IQR, 60–87.5 min).
Concomitant surgical manoeuvres other than biopsy were performed in 18 patients and included talc pleurodesis with or without pleural sampling (N=5), pericardial fenestration (N=2), and resection of an associated lung lesion (N=3). The 3 resected lung nodules were proven to be satellite tumor localization (N=1), aspergilloma (N=1), and a second primary tumor (N=1). In another patient with pericardial effusion, fenestration was not technically possible due to massive malignant adhesions to the lung resulting in poor visualization.
Postoperative results
Diagnostic yield was 100%. Definitive pathology included lymphomas in 13 patients, lung cancer in 9, thymic carcinoma and Castleman disease in one patient each. Perioperatively, adverse events occurred in 8 patients [33%, median Clavien-Dindo score: 0 (IQR, 0–1)]. There were just two grade 2 complications (high-frequency atrial fibrillation requiring pharmacological conversion and severe hypoxia requiring supplemental oxygen administration and radiographic confirmation of full lung expansion). Median hospital stay was 2 days (IQR, 2–3 days). Time interval to the oncological treatment was 7 days (IQR, 5.5–11.5 days).
Comparative results
Results of unmatched and propensity-matched comparison (8 patients a group) are displayed in Tables 2,3 respectively. As shown, differences in perioperative adverse events reached the significance threshold. In particular, in the matched general anesthesia group, there were two grade 4 complications—both consisting in prolonged ICU stay and continuous positive airway ventilation)—and one grade 3 event (phlegm retention and atelectasis requiring bronchoscopy). The global time spent in the OR was also shorter in the MVATS group. Time interval to oncological treatment was the same between groups. Median systemic-inflammation syndrome (SIRS) score and lowest measured PaO2/FiO2 ratio over 24-h observation—were better in the MVATS group.

Full table

Full table
Discussion
Surgical biopsy of patients with undetermined mediastinal tumors can prove challenging despite the technical easiness of the procedure, due to the potential for development of serious adverse events. In these instances the underlying pathophysiologic mechanisms can be most commonly related to reflex arrhythmias, bronchial spasm; compartmental pulmonary inflammation induced by one-lung ventilation and loss of airway patency and increased airway resistance resulting from deep sedation and curarization. All the aforementioned factors are assumed to be particularly more hazardous in patients with pre-existing compression of central airways and/or major vessels.
Based on the considerations above, we implemented MVATS for the treatment of patients with mediastinal tumors requiring surgical biopsy. In this decision, we were encouraged by the satisfactory results observed in other delicate subcohorts including patients undergoing awake lung volume reduction surgery for advanced emphysema, as well as patients with interstitial lung disease receiving MVATS biopsy (7,8).
The most relevant finding of the present study is that MVATS resulted safely applicable to patients with undetermined mediastinal tumors and have reduced the rate and severity of perioperative adverse events compared to equivalent procedures performed through general anesthesia and one-lung ventilation.
In recent years, mini-invasive approaches for mediastinal sampling have been a matter of innovation and investigational interest. Nonetheless, the establishment of clearly defined recommendations is still on debate. CT-guided fine-needle aspiration or core-biopsy has become increasingly popular, although there is certain discordance on the actual diagnostic accuracy of these techniques. In particular, regarding hematologic malignancy, Agid and colleagues (14) have reported a diagnostic yield of only 82.5%. Furthermore, in a prospective study by Feng et al. (15) comparing anterior mediastinotomy vs. CT-guided biopsy for mediastinal tumors, the latter resulted in a diagnostic yield of 41.7% only. Gossot and colleagues (16) reported a 75% failure rate with CT-guided biopsy in restaging residual lymphomas. Similar results have been confirmed even in more recent surveys (17). These figures substantially overlap that reported with endoscopic ultrasound techniques (18), given the similar amount of tissue that can be obtained in this way.
In general, there is a just limited experience on surgical management of mediastinal masses with local or locoregional anesthesia (19,20). A technique of anterior video-assisted mediastinotomy under local anesthesia has been reported (21) some years ago. However, this approach does not allow concomitant management of associated thoracic conditions other than the mediastinal tumors itself, and it is only available for tumors in the anterior mediastinum. Furthermore, this approach is carried out in supine position, which has been reported to increase the risk of airway compression (22). Finally, it can delay salvage radiotherapy due to the location of the skin incision, which falls in line with the irradiated field. In 2008, Matsumoto and colleagues (23) reported that MVATS can be applied to thymectomy for myasthenia gravis, even though no patient in that series had compressive effects on mediastinal structures secondary to huge thymic lesions.
We believe that advantages of MVATS biopsy over all the aforementioned surgical and non-surgical methods include increased safety, wide visual control of mediastinal sampling and accurate assessment of disease’s extension. As well, it offers the possibility of performing pleural-pulmonary biopsies and of draining concomitant pleural or pericardial effusion. In this respect, immediate management of pericardial effusion is not to be underrated, as this condition portrays an increased risk of poor outcome in patients with mediastinal tumors (2). Yet another advantage of MVATS over anterior mediastinotomy and CT-guided methods is that the former is performed in a lateral decubitus position, a fact that limits the risk of central airway compression (22).
All these considerations can have relevant practical implications. Indeed, in the era of targeted therapies, it is expected that an increasing number of surgical biopsies will be required to allow gene-expression analysis as well as more precise identification of “grey-zone” diseases. In these instances, MVATS biopsy may offer an adjunctive—and sometimes mandatory—diagnostic tool, other than allowing for the treatment of associated conditions.
As a result, the adoption of a uniportal approach in MVATS together with ongoing refinements of intraoperative analgesia protocols have contributed to further increase acceptance of surgical biopsy in this specific context.
Hence, MVATS offers now the opportunity to perform a safe and precisely targeted surgical biopsy of the tumor including also an accurate exploration of the pleural cavity or other surgical maneuvers through a single incision that is no greater than that required to insert a simple chest drain.
Limitations
The results of this study must be interpreted with caution due to the relatively small patient sample. A larger analysis is warranted to validate externally these preliminary observations and draw the actual pros and cons of MVATS in this specific patients subgroup. Further studies should also include some basic pathophysiological aspects of MVATS in general, such as preservation of perioperative immunosurveillance and reduced systemic inflammation. This kind of considerations were far beyond the goal of the present study, but their systematic analysis will help identify other potential advantages of MVATS over standard VATS in terms of both short- and long-term outcomes.
Conclusions
In our study, MVATS biopsy proved safely applicable to patients with undetermined mediastinal tumors and resulted in optimal diagnostic yield with no relevant adverse effect. In addition, comparisons with results of a matched control group showed that MVATS resulted in less perioperative adverse events and greater time-effectiveness compared to standard VATS under general anesthesia and one-lung ventilation.
Acknowledgements
We thank Prof. Tommaso Claudio Mineo, Professor and Head of the Department of Thoracic Surgery until November 1, 2015 for his pioneering contribution and encouragement in this research field.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study is a part of a broader investigational program of MVATS on approval of the Institutional Review Board of Tor Vergata University Hospital. Indication for surgical biopsy was established in the context of a multidisciplinary meeting and all patients signed a consent form after detailed discussion on expected benefits and drawbacks of the scheduled surgical plan.
References
- Goh MH, Liu XY, Goh YS. Anterior mediastinal masses: an anaesthetic challenge. Anaesthesia 1999;54:670-4. [Crossref] [PubMed]
- Azizkhan RG, Dudgeon DL, Buck JR, et al. Life-threatening airway obstruction as a complication to the management of mediastinal masses in children. J Pediatr Surg 1985;20:816-22. [Crossref] [PubMed]
- Béchard P, Létourneau L, Lacasse Y, et al. Perioperative cardiorespiratory complications in adults with mediastinal mass: incidence and risk factors. Anesthesiology 2004;100:826-34; discussion 5A.
- Pullerits J, Holzman R. Anaesthesia for patients with mediastinal masses. Can J Anaesth 1989;36:681-8. [Crossref] [PubMed]
- Prakash UB, Abel MD, Hubmayr RD. Mediastinal mass and tracheal obstruction during general anesthesia. Mayo Clin Proc 1988;63:1004-11. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54, 54.e1.
- Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg 2013;95:445-52. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Mineo D, et al. Awake nonresectional lung volume reduction surgery. Ann Surg 2006;243:131-6. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Fabbi E, et al. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg 2010;37:594-601. [Crossref] [PubMed]
- Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960-6. [Crossref] [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Pompeo E. Minimalistic thoracoscopic anterior spinal release in Scheuermann kyphosis. J Thorac Cardiovasc Surg 2013;146:490-1. [Crossref] [PubMed]
- Agid R, Sklair-Levy M, Bloom AI, et al. CT-guided biopsy with cutting-edge needle for the diagnosis of malignant lymphoma: experience of 267 biopsies. Clin Radiol 2003;58:143-7. [Crossref] [PubMed]
- Fang WT, Xu MY, Chen G, et al. Minimally invasive approaches for histological diagnosis of anterior mediastinal masses. Chin Med J (Engl) 2007;120:675-9. [PubMed]
- Gossot D, Girard P, de Kerviler E, et al. Thoracoscopy or CT-guided biopsy for residual intrathoracic masses after treatment of lymphoma. Chest 2001;120:289-94. [Crossref] [PubMed]
- Petranovic M, Gilman MD, Muniappan A, et al. Diagnostic yield of CT-guided percutaneous transthoracic needle biopsy for diagnosis of anterior mediastinal masses. AJR Am J Roentgenol 2015;205:774-9. [Crossref] [PubMed]
- Korrungruang P, Oki M, Saka H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration is useful as an initial procedure for the diagnosis of lymphoma. Respir Investig 2016;54:29-34. [Crossref] [PubMed]
- Katlic MR. Video-assisted thoracic surgery utilizing local anesthesia and sedation. Eur J Cardiothorac Surg 2006;30:529-32. [Crossref] [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [Crossref] [PubMed]
- Rendina EA, Venuta F, De Giacomo T, et al. Biopsy of anterior mediastinal masses under local anesthesia. Ann Thorac Surg 2002;74:1720-2; discussion 1722-3.
- Cho Y, Suzuki S, Yokoi M, et al. Lateral position prevents respiratory occlusion during surgical procedure under general anesthesia in the patient of huge anterior mediastinal lymphoblastic lymphoma. Jpn J Thorac Cardiovasc Surg 2004;52:476-9. [Crossref] [PubMed]
- Matsumoto I, Oda M, Watanabe G. Awake endoscopic thymectomy via an infrasternal approach using sternal lifting. Thorac Cardiovasc Surg 2008;56:311-3. [Crossref] [PubMed]