Prognostic factors in non-small cell lung cancer patients who received neoadjuvant therapy and curative resection
Introduction
Lung cancer is the leading cause of cancer deaths in the world, and more and more treatment modalities have been introduced in order to improve patients’ survival. Most patients with advanced non-small cell lung cancer (NSCLC), suffer disease relapse within three years and less than 10% of patients remain alive after a 5-year interval despite surgery (1,2). Because of poor survival, chemotherapy, radiotherapy and target therapy have been utilized in managing advanced NSCLC (3-6). From the literature review, neoadjuvant chemotherapy followed by surgical resection has been considered useful in select patients with advanced NSCLC, but the postoperative 5-year survival rates of these patients has ranged from 10% to 36% (1,7,8). In previous studies, the prognosis of patients with advanced NSCLC after neoadjuvant therapy has been based on the change in maximal standard uptake value (SUVmax) on fluorodeoxyglucose positron emission tomography (FDG-PET) scan, tumor size regression, lymph node (LN) status and clinical stage (9-12). However, these factors are usually evaluated preoperatively by radiologic imaging tools. Although chest tomography (CT) and FDG-PET scan provide more detailed information about disease severity, more and more studies have revealed significant differences between clinical and pathological stage (13-19). In patients who have received neoadjuvant therapy, occult metastases and alterations to the tumor microenvironment by chemotherapy may interfere with the FDG uptake and lead to a false negative result. This, in turn, would lead to discrepancy between clinical and pathologic stage and underestimation of the disease severity. The discrepancy would be the reason for poor survival prediction that has been reported by previous studies which conducted their analysis from the point of view of clinical stage (20-22). Therefore, the aim of this study was to analyze the relationship between clinico-pathologic factors and survival from the pathologic point of view and to try to identify survival prognostic factors.
Methods
Patients
From January 2005 to June 2011, a total of 609 patients received operations at Chang Gung Memorial Hospital. After exclusion, only 88 patients who had received neoadjuvant therapy because of initial locally advanced disease, and had subsequently undergone anatomic resection and mediastinal LN dissection were included in the study. Exclusion criteria included not receiving neoadjuvant therapy (442 patients), wedge resection due to poor pulmonary reserve (43 patients), small cell lung cancer (11 patients) and positive resection margin or TNM stage greater than IIIA (25 patients). All the clinico-pathologic data of the 88 included subjects were collected from a retrospective review of the medical records. The study was approved by the ethics committee of Chang Gung Memorial Hospital, under the Institutional Review Board number 103-5631B.
Neoadjuvant therapy and pre-operation restaging
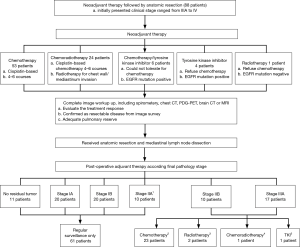
All patients initially presented as locally advanced disease based on complete image survey, with the clinical stage varying from IIIA to IV, before neoadjuvant therapy. Different types of neoadjuvant therapy were given according to patients’ status. The majority of patients (53%, 63.64%) received 4 to 6 courses of cisplatin based chemotherapy, depending on their general condition. Twenty-four patients (27.27%) received systemic chemotherapy and radiotherapy for local disease control because of mediastinum and chest wall invasion. Six patients (6.82%) received 3-month tyrosine kinase inhibitor therapy because of intolerance to cisplatin-based chemotherapy and tumor genetic survey positive for epidermal growth factor receptor (EGFR) mutation. A total of 5 patients (5.69%) refused cisplatin-based chemotherapy. One of them (1.14%) received radiotherapy only due to absence of EGFR mutation. A total of 4 patients (4.55%) received 3-month tyrosine kinase inhibitor therapy because of positive EGFR mutation result. After completion of neoadjuvant therapy, treatment response was re-evaluated by imaging tool, including chest CT, FDG-PET scan, and brain CT or magnetic resonance image (MRI). A revised clinical stage was given according to image evaluation result. The possible distant metastases were complete excluded by image evaluation.Only patients who presented as resectable disease from image survey, i.e., less than stage IIIA, received further anatomic resection and mediastinal LN dissection (Figure 1).
Operation
Patients who presented as resectable disease after neoadjuvant therapy underwent anatomic resection with mediastinal LN dissection 3 to 4 weeks after completion of neoadjuvant therapy. All procedures were performed via open thoracotomy or video assisted thoracoscopic surgery (VATS).
The corresponding pulmonary vein, artery, and bronchus were individually identified and divided with the aid of suture ligation or endoscopic staples. Subsequently, complete mediastinal LN dissection was performed. All resected specimens were examined by pathologist and the pathologic stages were classified according to American Joint Cancer Conference (AJCC) staging.
Post-operative treatment and follow-up
Post-operative adjuvant therapies were given according to the National Comprehensive Cancer Network (NCCN) guideline recommendations and pathologic stage. For patients with no residual tumor, i.e., stage IA and stage IB, only a close surveillance program was performed. In this study, 10 patients classified as stage IIA were recruited before 2009, and all presented with larger tumor size varying from 5 to 7 cm, but without mediastinal LN involvement. These patients were classified as stage IB in the 6th edition AJCC stage system and all were managed as stage IB without adjuvant therapy. Cisplatin-based chemotherapy was prescribed for patients if final pathologic stage was identified as stage II or higher.
Additional radiotherapy was given for adjuvant therapy if chest wall invasion was identified even with negative resection margin. However, if patients refused further adjuvant cisplatin-based chemotherapy, another alternative treatment, such as tyrosine kinase inhibitor or radiotherapy was given according to patients’ status (Figure 1). Patients were required to return to the outpatient department every three months, at which point a chest plain film or chest computed tomography was produced.
Statistical analysis
All collected clinico-pathologic factors were evaluated by univariate analysis. Categorical variables were compared using chi-square tests, while continuous variables were compared using two sample t-tests.
Disease free survival (DFS) was defined as no evidence of relapse in the period from the date of the operation to the last follow up date or the confirmation date of disease relapse. Overall survival (OS) was defined as the period between the operation date and death of any cause. The survival status was calculated by the Kaplan-Meier method, and the differences were analyzed by means of the log-rank test. A cox proportional hazards model was used to examine the multiple variables that were thought to be potential prognostic variables for survival in univariate analysis. A P value less than 0.05 was considered statistically significant. All analyses were performed using SAS, version 9 (SAS Institute, NC, USA).
Results
Patient characteristics
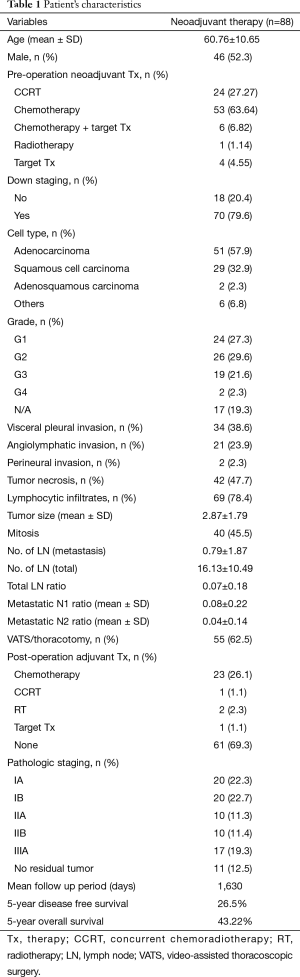
Eighty-eight patients with neoadjuvant therapy followed by anatomic resection were included in this study. The patient’s characteristics are shown in Table 1. The median age of patients was 60.76 years (±10.65) and 46 patients (52.3%) were male. Among these patients, 53 patients (63.64%) received chemotherapy, 24 patients (27.27%) received chemo-radiotherapy, 6 patients (6.82%) received a combination of chemotherapy and tyrosine kinase inhibitor, 4 patients (4.55%) had target therapy, and 1 patient (1.14%) received radiotherapy. Seventy patients (79.6%) were found to be down stage from the image survey before operation. The cell types at final diagnosis showed 51 patients (57.9%) with adenocarcinoma, 29 patients (32.9%) with squamous cell carcinoma, 2 patients (2.3%) with adenosquamous carcinoma and 6 patients (6.8%) with other cell types.
Full table
Surgical outcomes and adjuvant therapy
Fifty-five patients (62.5%) underwent VATS. Pathological stage distribution was 20 patients (22.3%) with stage Ia, 20 patients (22.7%) with stage Ib, 10 patients (11.3%) with stage IIa, 10 patients (11.4%) with stage IIb, and 17 patients (19.3%) with stage IIIa. The mean tumor size was 2.87 (±1.79) cm and 11 patients (12.5%) were found with no viable residual tumor. Visceral pleural invasion, angiolymphatic invasion and perineural invasion were found in 34 patients (38.6%), 21 patients (23.9%) and 2 patients (2.3%) respectively. Forty-two patients (47.7%) were found to have tumor necrosis and 69 patients (78.4%) were found to have lymphocytic infiltration. The mean number of retrieved LNs was 16.13 (±10.49) and the mean number of metastastic LN was 0.79 (±1.87). Total metastatic LN ratio was 0.07 (±0.18), of which metastatic N1 LN ratio was 0.08 (±0.22) and metastatic N2 LN ratio was 0.04 (±0.14). Sixty-one patients (69.3%) received regular surveillance because of no residual tumor or stage I disease (Figure 1). Twenty-three patients (26.1%) received adjuvant cisplatin-based chemotherapy. Adjuvant chemo-radiation was given for 2 patients (2.3%). Additional radiotherapy was applied in 1 patient (1.1%), and tyrosine kinase was applied in 1 patient (1.1%).
Survival and prognostic factor analysis
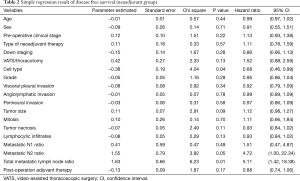
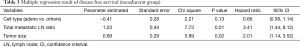
The median follow-up period for all patients was 1,630 days. Five-year DFS and OS were 26.5% and 43.22%, respectively. The univariate and multivariate analysis of DFS in all patients are shown in Tables 2 and 3, respectively. In the univariate analysis, cell type (P=0.04) and total metastatic LN ratio (P=0.01) were found to have a significant impact on DFS. Tumor size showed a trend toward significance (P=0.09). In the multivariate analysis, we found that total metastatic LN ratio (P=0.01) and tumor size (P=0.02) were predictive factors for DFS. We found a tumor size of 5 cm and total metastatic LN ratio at 0.065 to be the threshold values with regard to DFS (Figure 2A,B) and further applied these two prognostic factors for stratification. All patients were sub-grouped into four groups by these two factors (Figure 2C). Group 4 (tumor size ≤5, total metastatic LN ratio ≤0.065) had the best DFS curve, while the DFS curve progressively deteriorated through group 3 (tumor size ≤5, total metastatic LN ratio >0.065), group 2 (tumor size >5, total metastatic LN ratio ≤0.065) and group 1 (tumor size >5, total metastatic LN ratio >0.065). In addition, the more poor prognostic factors were identified, the higher risk of disease relapse were noted (Figure 2D).
Full table
Full table
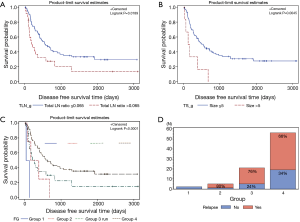
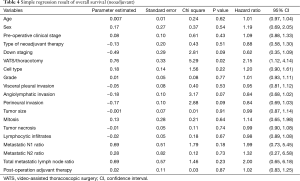
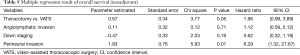
The univariate and multivariate analyses of OS in all patients are shown in Tables 4 and 5, respectively. In the univariate analysis, operative method (P=0.02) was found to have significant impact on OS. Down staging (P=0.09), angiolymphatic invasion (P=0.07) and perineural invasion (P=0.09) were found to have a trend toward significance. In the multivariate analysis, we identified only perineural invasion (P=0.01) as a predictive factor for OS. Further investigation is warranted because only two patients were identified as having perineural invasion. In addition, no definite prognostic factor could be identified in this study.
Full table
Full table
Discussion
In this study, we tried to find predictive prognostic factors in patients with advanced NSCLC after neoadjuvant therapy followed by surgical resection. Our study included patients who initially presented in clinical stage varied from IIIA to IV before neoadjuvant treatment. Patients who presented as clinical stage IIIA showed similarity to those who presented with IIIB and IV because of the possibility of occult metastases. The small occult metastases may be hidden in successive slice of computed tomography and may not appear in positron-emission tomography (23). The only difference between stage IIIA and other advance stage, including IIIB and IV, was microscopic and macroscopic metastasis, respectively. From NCCN guideline, surgical resection may be beneficial for these patients who presented with fore-mentioned scenarios that similar to those presented clinical stage IIIA (24). In addition, all patients who presented as resectable disease in tumor re-evaluation after neoadjuvant therapy were underwent anatomical resection and mediastinal LN dissection. Our study included all advanced NSCLC patients with similar presentation and those who may be beneficial from neoadjuvant therapy followed by surgical curative resection which was differ than other literatures. Our result revealed that pathological tumor size and total metastatic LN ratio are important prognostic factors with regard to DFS. In this study, we not only clarified that tumor size and metastatic LN ratio are correlated to DFS, but we also quantified these two factors, in particular, a tumor size greater than 5 cm and total metastatic LN ratio greater than 0.065, based on the pathological findings. Patients with tumor size ≤5 cm and total metastatic LN ratio ≤0.065 had the most sustained DFS, compared to those with tumor size >5 cm and total metastatic LN ratio >0.065. Furthermore, we found that tumor size plays a more important role than total metastatic LN ratio with regard to DFS. However, no definite prognostic factor was identified regarding OS except perineural invasion. From the literature review, the role of perineural invasion remains controversial (25,26). In our study, perineural invasion was identified as a prognostic factor regarding OS, but further investigation is warranted due to the limited number of cases.
For NSCLC patients who received neoadjuvant therapy, tumor down staging after evaluation by imaging tools was important for resectability evaluation. However, the discrepancy between clinical stage and pathologic stage was demonstrated with the agreement rate at around 35% (16,18). In addition, neoadjuvant therapy is thought to interfere with the interpretation of examination results (21). This leads not only to a lowered agreement rate between clinical and pathologic stage, but also less survival predicting power for DFS and OS (27). From the literature review, many prognostic factors have been identified for patients who have been treated with neoadjuvant therapy based on pathology findings. Metastatic LN ratio, number of residual metastatic LNs, smaller area of residual tumor (less than 400 mm2) and negative pleural invasion, percentage of viable residual tumor cells, and low total macrophage number in the tumor have been correlated with survival in patients who have received neoadjuvant therapy and subsequent surgical resection (28-38). In this study, we identified tumor size larger than 5 cm and total metastatic lymph ratio less than 0.065 as correlated to DFS. This finding is similar to that of previous studies, but much easier for clinical application. We did not have to measure tumor volume, calculate viable tumor cell percentage, calculate the total macrophage number in the tumor area, or elaborate further elastin stain for visceral pleura invasion confirmation. All of these measurements may be vulnerable to bias between different pathologists. Our result was obtained through a quite simple measurement that minimized observational bias. In addition, factors correlating to disease invasion status, i.e., tumor size and metastatic LN were included that could be more precise in survival prediction. Our result could help clinicians set up individually tailored follow-up programs and treatment strategies for patients with advanced NSCLC after neoadjuvant therapies followed by surgical resection. More aggressive post-operation adjuvant and maintenance therapy should be considered when patients are identified with one or two of the prognostic factors, and individualized follow up programs should be planned if needed. However, further investigation is warranted to clarify the real survival impact mechanism.
There are some limitations to our study. First, this study was conducted as a retrospective review.
Second, the sample size of this study was too small to stratify patients into different subgroups resulting in unreliable parameter validation. Third, different types of neoadjuvant and post-operation adjuvant therapy were used for these patients and we could differentiate the effect on survival in this study. However, because of the small sample size, further investigation should be conducted to validate the predictive values of tumor size and total metastatic LN ratio. Although limitations remain, out study was able to stratify patients treated with neoadjuvant therapy followed by surgical resection into different subgroups. Patients with tumor size greater than 5 cm and metastatic LN ratio greater than 0.065 revealed extremely poor DFS and aggressive adjuvant therapy should be considered.
Conclusions
In conclusion, tumor size greater than 5 cm and total metastatic LN ratio greater than 0.065 can predict the DFS of patients with advanced NSCLC after multimodality therapies followed by surgical resection. Tumor size plays a more important role than total metastatic LN ratio on DFS. Moreover, patients who are identified with these factors need aggressive post-operation surveillance and additional aggressive adjuvant therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of Chang Gung Memorial Hospital, under the Institutional Review Board number 103-5631B.
References
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Santo A, Genestreti G, Sava T, et al. Neo-adjuvant chemotherapy in non-small cell lung cancer (NSCLC). Ann Oncol 2006;17 Suppl 5:v55-61. [Crossref] [PubMed]
- Felip E, Cedrés S, Checa E, et al. How to integrate current knowledge in selecting patients for first line in NSCLC? Ann Oncol 2010;21 Suppl 7:vii230-3. [Crossref] [PubMed]
- Cho JH, Kim J, Kim K, et al. Risk associated with bilobectomy after neoadjuvant concurrent chemoradiotherapy for stage IIIA-N2 non-small-cell lung cancer. World J Surg 2012;36:1199-205. [Crossref] [PubMed]
- Zhai H, Zhong W, Yang X, et al. Neoadjuvant and adjuvant epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy for lung cancer. Transl Lung Cancer Res 2015;4:82-93. [PubMed]
- Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol 2015;16:395-405. [Crossref] [PubMed]
- Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer 2005;50:227-34. [Crossref] [PubMed]
- Horita N, Miyazawa N, Morita S, et al. Preoperative chemotherapy is effective for stage III resectable non--small-cell lung cancer: metaanalysis of 16 trials. Clin Lung Cancer 2013;14:488-94. [Crossref] [PubMed]
- Weber WA, Petersen V, Schmidt B, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol 2003;21:2651-7. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Winokur TS, et al. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg 2004;78:1903-9. [Crossref] [PubMed]
- Eschmann SM, Friedel G, Paulsen F, et al. Is standardised (18)F-FDG uptake value an outcome predictor in patients with stage III non-small cell lung cancer? Eur J Nucl Med Mol Imaging 2006;33:263-9. [Crossref] [PubMed]
- Tieu BH, Sanborn RE, Thomas CR. Neoadjuvant therapy for resectable non-small cell lung cancer with mediastinal lymph node involvement. Thorac Surg Clin 2008;18:403-15. [Crossref] [PubMed]
- Gwyther SJ. Current standards for response evaluation by imaging techniques. Eur J Nucl Med Mol Imaging 2006;33 Suppl 1:11-5. [Crossref] [PubMed]
- Subedi N, Scarsbrook A, Darby M, et al. The clinical impact of integrated FDG PET-CT on management decisions in patients with lung cancer. Lung Cancer 2009;64:301-7. [Crossref] [PubMed]
- Santos PA, Rocha RS, Pipkin M, et al. Concordance between clinical and pathological staging in patients with stages I or II non-small cell lung cancer subjected to surgical treatment. J Bras Pneumol 2007;33:647-54. [Crossref] [PubMed]
- Younes RN, Schutz FA, Gross JL. Preoperative and pathological staging of NSCLC: retrospective analysis of 291 cases. Rev Assoc Med Bras 2010;56:237-41. [Crossref] [PubMed]
- Turk F, Gursoy S, Yaldiz S, et al. Comparison of clinical and pathological tumor, node and metastasis staging of lung cancer: 15-year experience with 530 patients. Minerva Chir 2011;66:509-16. [PubMed]
- Muehling B, Wehrmann C, Oberhuber A, et al. Comparison of clinical and surgical-pathological staging in IIIA non-small cell lung cancer patients. Ann Surg Oncol 2012;19:89-93. [Crossref] [PubMed]
- Li S, Zheng Q, Ma Y, et al. Implications of false negative and false positive diagnosis in lymph node staging of NSCLC by means of 18F-FDG PET/CT. PLoS One 2013;8:e78552. [Crossref] [PubMed]
- Port JL, Kent MS, Korst RJ, et al. Positron emission tomography scanning poorly predicts response to preoperative chemotherapy in non-small cell lung cancer. Ann Thorac Surg 2004;77:254-9; discussion 259. [Crossref] [PubMed]
- Rebollo-Aguirre AC, Ramos-Font C, Villegas Portero R, et al. Is FDG-PET suitable for evaluating neoadjuvant therapy in non-small cell lung cancer? Evidence with systematic review of the literature. J Surg Oncol 2010;101:486-94. [PubMed]
- William WN Jr, Pataer A, Kalhor N, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2013;8:222-8. [Crossref] [PubMed]
- Kappers I, van Sandick JW, Burgers JA, et al. Results of combined modality treatment in patients with non-small-cell lung cancer of the superior sulcus and the rationale for surgical resection. Eur J Cardiothorac Surg 2009;36:741-6. [Crossref] [PubMed]
- Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Chang PM, Yeh YC, Chen TC, et al. High expression of CHRNA1 is associated with reduced survival in early stage lung adenocarcinoma after complete resection. Ann Surg Oncol 2013;20:3648-54. [Crossref] [PubMed]
- Yilmaz A, Duyar SS, Cakir E, et al. Clinical impact of visceral pleural, lymphovascular and perineural invasion in completely resected non-small cell lung cancer. Eur J Cardiothorac Surg 2011;40:664-70. [PubMed]
- Lee H, Ahn YC, Pyo H, et al. Pretreatment clinical mediastinal nodal bulk and extent do not influence survival in N2-positive stage IIIA non-small cell lung cancer patients treated with trimodality therapy. Ann Surg Oncol 2014;21:2083-90. [Crossref] [PubMed]
- Martini N, Burt ME, Bains MS, et al. Survival after resection of stage II non-small cell lung cancer. Ann Thorac Surg 1992;54:460-5;discussion 466. [Crossref] [PubMed]
- Sawyer TE, Bonner JA, Gould PM, et al. Factors predicting patterns of recurrence after resection of N1 non-small cell lung carcinoma. Ann Thorac Surg 1999;68:1171-6. [Crossref] [PubMed]
- Osaki T, Nagashima A, Yoshimatsu T, et al. Survival and characteristics of lymph node involvement in patients with N1 non-small cell lung cancer. Lung Cancer 2004;43:151-7. [Crossref] [PubMed]
- Li ZM, Ding ZP, Luo QQ, et al. Prognostic significance of the extent of lymph node involvement in stage II-N1 non-small cell lung cancer. Chest 2013;144:1253-60. [Crossref] [PubMed]
- Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg 2012;93:1614-9; discussion 1619-20. [Crossref] [PubMed]
- Wisnivesky JP, Arciniega J, Mhango G, et al. Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer.Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer. Thorax 2011;66:287-93. [Crossref] [PubMed]
- Kim SH, Cho BC, Choi HJ, et al. The number of residual metastatic lymph nodes following neoadjuvant chemotherapy predicts survival in patients with stage III NSCLC. Lung Cancer 2008;60:393-400. [Crossref] [PubMed]
- Yamane Y, Ishii G, Goto K, et al. A novel histopathological evaluation method predicting the outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor. J Thorac Oncol 2010;5:49-55. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Feng PH, Yu CT, Wu CY, et al. Tumor-associated macrophages in stage IIIA pN2 non-small cell lung cancer after neoadjuvant chemotherapy and surgery. Am J Transl Res 2014;6:593-603. [PubMed]
- Lim HJ, Lee HY, Lee KS, et al. Predictive factors for survival in stage IIIA N2 NSCLC patients treated with neoadjuvant CCRT followed by surgery. Cancer Chemother Pharmacol 2015;75:77-85. [Crossref] [PubMed]


