Clinical outcomes of cytoreductive surgery combined with intrapleural perfusion of hyperthermic chemotherapy in advanced lung adenocarcinoma with pleural dissemination
Introduction
Although the official standard treatment for advanced lung cancers is concurrent chemo-radiation therapy, various therapeutic strategies have been attempted and are considered acceptable (1). For example, cases of stage IV lung cancer with solitary distant metastasis are candidates for curative surgical resection (2). In order words, certain carefully selected patients with recurrent lung cancers can expect to be cured by surgical methods (3). Along these same lines, an unexpectedly good prognosis has been reported in non-small cell lung cancer (NSCLC) patients with minimal carcinomatous pleuritis (4), although surgery alone is not considered a suitable choice for NSCLC with pleural dissemination. In other words, multimodal therapeutic approaches combining surgery, chemotherapy, radiotherapy, targeted therapy, or supportive care may all be beneficial for treating advanced-stage lung cancer (5).
Intrapleural perfusion hyperthermic chemotherapy (IPHC) has been applied to control malignant pleural effusion (6). Extensive surgical resection combined with IPHC is a well-known surgical approach for malignant pleural mesothelioma (7,8), and has been used to treat thymic carcinoma with pleural dissemination (9). Promising results of surgical resection combined with IPHC for lung cancer with pleural dissemination have also been reported (10,11).
We have performed IPHC 84 times since 2003 for various thoracic pleural malignancies. This experience has included 23 cases of lung adenocarcinoma with pleural dissemination treated with surgical resection of primary lesions combined with IPHC. During the same time period we have seen 10 patients with lung adenocarcinoma and pleural dissemination who were treated by surgical resection but not IPHC. In the present study, we analyzed the medical records of these patients and compared perioperative outcomes to investigate the safety and feasibility of IPHC combined with surgery.
Methods
Patients
We performed a retrospective review of medical records of lung adenocarcinoma patients who had undergone surgical resections between March 2003 and December 2013. During this period, 1,279 patients underwent curative surgical resection for lung adenocarcinoma, from which we identified 33 cases of pleural dissemination (M1a). The presence of pleural seeding in these 33 patients was confirmed pathologically during or after surgery. In 11 cases, pleural seeding was identified incidentally during surgery, while the rest of the cases were suspicious on imaging studies. None of the cases were pathologically confirmed until surgery. This study was approved by Institutional Review Board of Seoul National University Bundang Hospital (IRB number; B-1602-334-112). And all participants gave their informed consent before taking part.
Among the patients analyzed in this study, 23 underwent IPHC after surgical resection. Ten patients did not undergo IPHC for various reasons including patient refusal, preference of the surgeon, or grossly complete removal of metastatic lesions. Finally, we categorized these 23 patients in the IPHC group, while the remaining patients were assigned to the surgery group.
Treatment
All of the patients enrolled in this study underwent surgical resection for their primary lung lesions, either wedge resection or lobectomy. Lobectomy was the standard surgical resection procedure, while wedge resections were performed to reduce tumor burdens and confirm the precise characteristics of the cancer when lobectomies were considered to provide no additional advantages.
The protocols for IPHC started 30 minutes before intrapleural administration of perfusate. Specifically, 150 mL of a 15% mannitol solution was administered intravenously for 30 minutes. Chlorpheniramine and dolasetron mesylate were also administered. Patients considered to be at a high risk for acute renal injury were hydrated overnight with 1.5 L normal saline for 12 hours. In addition, intravenous administration of 0.2 mg/kg dexamethasone was considered the day before IPHC depending on the patient.
The perfusate consisted of 150 mg/m2 of cisplatin mixed with 3 L of normal saline. The temperature of the solution was controlled between 43 and 45 °C. The perfusate was circulated through chest tube tubes connected in a closed circuit with a roller pump for 90 minutes (Figure 1). During circulation, electrocardiography, arterial blood pressure, hourly urine output, and both rectal and esophageal temperature were monitored. The rectal temperature was kept lower than 38 °C, while that of the esophagus was kept lower than 40 °C. If the temperature exceeded those limitations, the circulation of perfusate was stopped to avoid injury to intrathoracic organs.
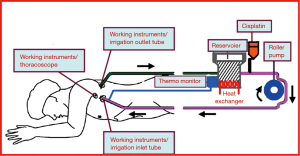
After complete drainage of the perfusate, an additional 150 mL of the 15% mannitol solution was administered for 30 minutes followed by sodium thiosulfate for 6 hours (4 g/m2 for 10 minutes, and 12 g/m2 for 6 hours). Infusion of 1 L of normal saline or Hartmann’s solution was considered on a per-patient basis during the first 12 hours postoperative, as was administration of Lasix in order to maintain an hourly urine output greater than 100 mL and prevent acute renal injury. Patients were given oral dexamethasone for 3 days, and laboratory results were monitored through postoperative day 5. All of the enrolled patients underwent adjuvant chemotherapy with the exception of one patient who died on postoperative day 15.
Statistical analysis
Patient characteristics of the IPHC group and surgery group were compared. Continuous data were analyzed by analysis of variance (ANOVA) and described as the mean ± standard deviation (SD). The χ2-test was performed for categorical variance. Loco-regional recurrence was defined as any suspicious finding on imaging studies at the surgical resection site, ipsilateral lung parenchyma, hilum or mediastinum, while all other sites were considered distant metastases. Disease progression was defined as identification of any finding related to locoregional or distant metastasis on follow-up examination including chest CT or PET-CT. We considered increasing pleural nodules, increasing sizes or pleural effusion in the chest CT during the follow-up period as evidence of progression of pleural dissemination.
The 6-month and 1-, 2-, and 3-year overall survival and disease progression-free survival rates were calculated using the Kaplan-Meier method, and survival between the IPHC and surgery groups was compared by log-rank test. All P values less than 0.05 with a 95% confidential interval were considered statistically significant. All statistical analyses were performed using SPSS 20.0 (SPSS Inc., Armonk, NY, USA).
Results
Preoperative characteristics
The mean follow-up periods were 34.5, 34.9 (±21.34, range, 0.5 to 99.9) months and the mean age was 62.1 (±7.21, range, 47 to 73) years. There were no statistically significant differences between the two groups with respect to age, sex, smoking history, N stage, presence of EGFR mutations, and use of neoadjuvant chemotherapy. However, T stage was significantly different (P=0.035) between the two groups. The characteristics of patients and their histopathologic characteristics are described in Tables 1,2.
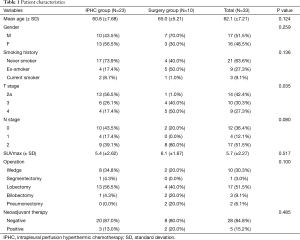
Full table
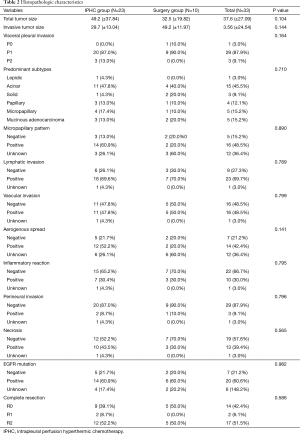
Full table
Perioperative outcomes
The mean interval periods for IPHC after surgery were 2.78 days (±3.06, range, 0 to 9), and there were 11 instances of IPHC performed concurrently with surgery. The most commonly performed surgical method was lobectomy (17 cases, 51.5%). The chest tube indwelling time of the IPHC group was significantly longer than that of the surgery group (P=0.010); however, the length of hospital stay and complication rates between the two groups were not different to a statistically significant degree. In the surgery group, one patient who underwent pneumonectomy died as a result of postoperative complications (acute respiratory distress syndrome). Perioperative outcomes are described in Table 3.
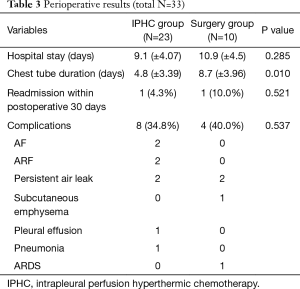
Full table
Survival analysis
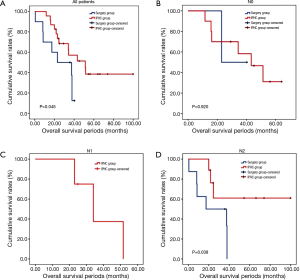
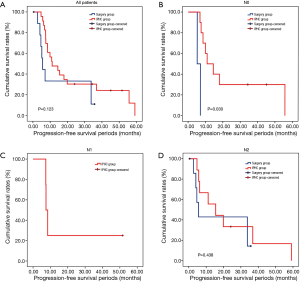
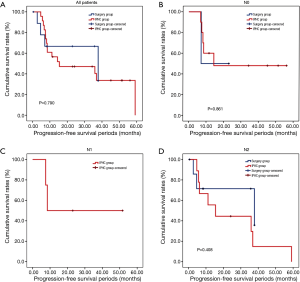
The overall survival rate of the IPHC group was significantly longer than that of the surgery group (P=0.045). The progression-free survival and pleural progression-free survival rates were not significantly different between the two groups. The survival analysis is summarized in Table 4, and survival curves are described in Figures 2-4.
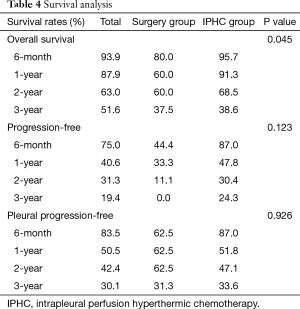
Full table
Discussion
There continues to be an ongoing debate regarding the most appropriate strategy for advanced lung cancer and NSCLC patients with isolated distant metastases that may be candidates for surgical management (2). In cases where the cancer is associated with pleural dissemination or malignant pleural effusion, IPHC with or without cytoreductive surgery is a suitable treatment option (10,11). Signs of pleural seeding are a poor prognosis factor; however, patients with minimal pleural dissemination identified during surgery have been reported to have relatively good surgical outcomes compared with other stage IV lung cancer patients (4).
Patients with NSCLC with pleural dissemination may be candidates for surgery in cases where pleural dissemination is first detected during the surgery or when the mediastinal lymph node is not positive (12). In these cases, surgical treatment alone is not expected to be successful, and other treatment modalities including chemotherapy, radiotherapy, or even palliative supportive care should be considered. For example, postoperative IPHC has been shown to be effective in controlling malignant pleural effusion in stage IV M1a, and has been applied in some cases with curative intent. Shigemura et al. reported the clinical results of five patients who had undergone panpleuropneumonectomy combined with IPHC, and concluded that IPHC is a valid surgical option for local tumor control as part of a multimodal treatment plan (13).
In the present study, we hypothesized that IPHC might have a positive influence on long-term survival of advanced lung adenocarcinoma patients with pleural dissemination whose primary lesion could be removed surgically. By performing a retrospective review of the medical records of 1,278 patients who underwent surgical treatment for lung adenocarcinomas, we identified 33 patients with pleural seeding confirmed by pathology during the operation. Among these patients, 23 received additional IPHC.
IPHC is commonly performed using cisplatin, and cisplatin is currently considered the most effective agent for controlling NSCLC (14). During the circulation of perfusate, serum cisplatin should be maintained at lower levels than that of chemotherapy; however, it is associated with risks of complications such as atrial fibrillation, pneumonia, and acute renal injury (15). To lower the systemic effects of perfusate and improve the anticancer effects, clinical trials of IPHC therapy related to other chemical agents have been studied (6,16). Although no standard guidelines have been established, careful protocols for peri- and postoperative care are necessary in order to prevent cisplatin-induced complications. Typically, blood laboratory tests are performed for five days postoperatively to detect any abnormalities, and renal protective agents are often applied. In order to protect intrathoracic organs, the temperature of perfusate should be controlled between 43 and 45 °C, and circulation of perfusate should be terminated if the esophageal temperature reaches higher than 40 °C.
Lung cancer patients with pleural disseminations are fragile due to the risk of distant metastasis (13). In these patients, adjuvant chemotherapy is essential. All of the patients included in this study underwent postoperative adjuvant chemotherapy, with the exception of one patient who died at postoperative day 15.
In comparison with clinical data from patients who did not receive IPHC as an additional treatment, we identified some benefits of IPHC. Specifically, overall survival was significantly longer (Figure 2), and in the N0 condition, progression-free survival was also significantly longer in the IPHC group (Figure 3). Remarkably, N2 patients in the IPHC group in our study showed survival benefits (Figure 2). This was notable because previous studies concluded that IPHC is beneficial for stage M1a with N0–1 patients (17). Although our study was confined to lung adenocarcinoma, our results may suggest the possibility that IPHC is beneficial for locally advanced lung cancers with pleural dissemination. Lastly, although the length of chest tube indwelling was longer in the IPHC group, other perioperative results including hospital stay and complication rates were not inferior to those of the surgery group.
The survival benefit of IPHC combined with surgical removal for advanced lung adenocarcinoma over the surgery only was somewhat doubtful, because the patient characteristics of the two groups were not homogenous. Although all patients were in stage IV M1a category, more advanced T4 stage (50% in surgery group and 17% in IPHC group), and N2 stages (80%, and 39% respectively). Two patients in surgery group had received pneumonectomy, whereas no one in IPHC group. There was a possibility that patients with less advanced cancers were chosen for the IPHC, or more aggressive surgical treatment had been performed for the advanced cancers in Surgery group. The observed survival benefit would be due to the patient selection bias.
However, the IPHC group showed significantly higher progression-free survival in N0 patients and overall survival in N2 patients. This might be the reflection that the IPHC could be used for local control as well as systemic effects (18).
There were other limitations to our study. First, there were no established guidelines for application of IPHC when pleural metastases were identified during surgery. In addition, comorbidities of patients and the extent of surgery may have influenced our results and contributed to a bias in patient selection. Furthermore, it would have been ideal to compare clinical results with those of patients treated with chemotherapy alone to establish the effectiveness of surgical methods. Unfortunately, the study population was too small to confirm a beneficial effect, and also this was retrospective study by design. In the future, more precisely designed prospective studies with a larger study population will be needed to determine the effectiveness of IPHC combined with surgical resection in patients with stage IV M1a lung adenocarcinoma.
In conclusion, our study showed that IPHC in addition to surgery for advanced lung adenocarcinoma with pleural dissemination could be performed with an acceptable rate of complications. It would be part of multimodality therapy for advanced lung adenocarcinoma, however, the long-term benefits for survival is uncertain. Although this study would be first research reported the clinical results for the IPHC combined with cytoreductive surgery in advanced lung adenocarcinoma, for more obvious and scientific evidences, we need to establish detailed inclusion criteria for enrolled patients and treatment guideline. More extensive and precisely designed studies are warranted to further evaluate the effectiveness of IPHC.
Acknowledgements
Funding: This work was supported by grant number 02-2008-021 from Seoul National University Bundang Hospital Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board of Seoul National University Bundang Hospital (No. B-1602-334-112) and written informed consent was obtained from all patients.
References
- Schreiner W, Dudek W, Lettmaier S, et al. Should salvage surgery be considered for local recurrence after definitive chemoradiation in locally advanced non-small cell lung cancer? J Cardiothorac Surg 2016;11:9. [Crossref] [PubMed]
- Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer 2010;69:251-8. [Crossref] [PubMed]
- Varela G, Thomas PA. Surgical management of advanced non-small cell lung cancer. J Thorac Dis 2014;6 Suppl 2:S217-23. [PubMed]
- Ichinose Y, Tsuchiya R, Koike T, et al. Prognosis of resected non-small cell lung cancer patients with carcinomatous pleuritis of minimal disease. Lung Cancer 2001;32:55-60. [Crossref] [PubMed]
- Simmons CP, Koinis F, Fallon MT, et al. Prognosis in advanced lung cancer--A prospective study examining key clinicopathological factors. Lung Cancer 2015;88:304-9. [Crossref] [PubMed]
- Matsuzaki Y, Shibata K, Yoshioka M, et al. Intrapleural perfusion hyperthermo-chemotherapy for malignant pleural dissemination and effusion. Ann Thorac Surg 1995;59:127-31. [Crossref] [PubMed]
- Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7. [Crossref] [PubMed]
- Migliore M, Calvo D, Criscione A, et al. Cytoreductive surgery and hyperthermic intrapleural chemotherapy for malignant pleural diseases: preliminary experience. Future Oncol 2015;11:47-52. [Crossref] [PubMed]
- Yu L, Jing Y, Ma S, et al. Cytoreductive surgery combined with hyperthermic intrapleural chemotherapy to treat thymoma or thymic carcinoma with pleural dissemination. Onco Targets Ther 2013;6:517-21. [Crossref] [PubMed]
- Kimura M, Tojo T, Naito H, et al. Effects of a simple intraoperative intrathoracic hyperthermotherapy for lung cancer with malignant pleural effusion or dissemination. Interact Cardiovasc Thorac Surg 2010;10:568-71. [Crossref] [PubMed]
- Işık AF, Sanlı M, Yılmaz M, et al. Intrapleural hyperthermic perfusion chemotherapy in subjects with metastatic pleural malignancies. Respir Med 2013;107:762-7. [Crossref] [PubMed]
- Mordant P, Rivera C, Legras A, et al. Current readings: the most influential and recent studies regarding resection of lung cancer in m1a disease. Semin Thorac Cardiovasc Surg 2013;25:251-5. [Crossref] [PubMed]
- Shigemura N, Akashi A, Ohta M, et al. Combined surgery of intrapleural perfusion hyperthermic chemotherapy and panpleuropneumonectomy for lung cancer with advanced pleural spread: a pilot study. Interact Cardiovasc Thorac Surg 2003;2:671-5. [Crossref] [PubMed]
- Du N, Li X, Li F, et al. Intrapleural combination therapy with bevacizumab and cisplatin for non-small cell lung cancer-mediated malignant pleural effusion. Oncol Rep 2013;29:2332-40. [PubMed]
- Zellos L, Richards WG, Capalbo L, et al. A phase I study of extrapleural pneumonectomy and intracavitary intraoperative hyperthermic cisplatin with amifostine cytoprotection for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2009;137:453-8. [Crossref] [PubMed]
- Yoshida K, Sugiura T, Takifuji N, et al. Randomized phase II trial of three intrapleural therapy regimens for the management of malignant pleural effusion in previously untreated non-small cell lung cancer: JCOG 9515. Lung Cancer 2007;58:362-8. [Crossref] [PubMed]
- Okamoto T, Iwata T, Mizobuchi T, et al. Pulmonary resection for lung cancer with malignant pleural disease first detected at thoracotomy. Eur J Cardiothorac Surg 2012;41:25-30. [PubMed]
- Ried M, Potzger T, Braune N, et al. Local and systemic exposure of cisplatin during hyperthermic intrathoracic chemotherapy perfusion after pleurectomy and decortication for treatment of pleural malignancies. J Surg Oncol 2013;107:735-40. [Crossref] [PubMed]


