Current status and problems of lung transplantation in Japan
Introduction
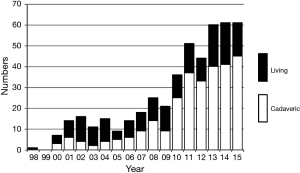
Lung transplantation has been performed successfully worldwide since 1983 in patients with end-stage lung disease, and more than 50,000 lung transplants have been reported in The Registry of the International Society for Heart and Lung Transplantation (1). In contrast, the history of lung transplantation in Japan has had a long dark period because of the difficulty in accepting the concept of brain death. The transplant law finally became effective in October 1997 and cadaveric lung transplantation (CLT) was officially approved. However, it was somewhat a very strict law resulting in very small practice of CLTs for the next decade. The author performed the first successful lung transplantation using living donors in 1998 at Okayama University (2). Living-donor lobar lung transplantation (LDLLT) had been the only realistic option for most patients until 2010 when the Japanese Organ Transplant Law was amended so that the family of the brain dead donors can make a decision for organ donation (3,4). The revision of the law significantly increased the number of organ donations from brain dead donors and CLT has become a more realistic option for adult Japanese patients since then (5). About 60 lung transplants, two thirds as CLT and one third as LDLLT had been performed annually during the last 3 years (Figure 1).
Cadaveric lung transplant system in Japan
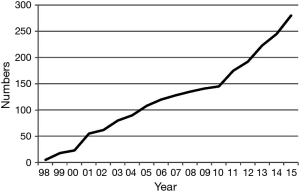
There are nine certified lung transplant centers in Japan (Tohoku, Dokkyo, Chiba, Tokyo, Kyoto, Osaka, Okayama, Fukuoka and Nagasaki Universities). Following multidisciplinary assessment, a decision is made to accept or reject the patient as a potential lung transplant recipient by the Lung Transplant Evaluation Committee at each lung transplant center. Candidates accepted by the Committee are evaluated further by nationwide Central Lung Transplant Evaluation Committee. Then, the accepted candidates are registered on the waiting list for CLT of the Japan Organ Transplant Network (JOTN). The number of lung transplant candidates newly registered at JOTN has generally been increasing. Accordingly, the number of patients on the waiting list is also increasing (Figure 2). Currently, about 300 patients are listed, however, only about 40 patients can receive CLT annually. The algorithm for brain dead donor lung allocation is based primarily on accrued time on the waiting list, a situation which favors patients with slowly progressive diseases and disadvantages patients with rapidly progressive diseases. As a result, the average waiting time is still more than 800 days resulting in nearly 50% mortality rate on the waiting list.
Indications
Lung transplantation is indicated for patients with end-stage lung disease who are failing maximal medical therapy and whose life expectancy is limited. Because of the severe cadaveric donor shortage, upper age limit is set in Japan. Candidates should be less than 55 years old for bilateral lung transplantation and they should be less than 60 years old for single lung transplantation at the time of registration on JOTN waiting list.
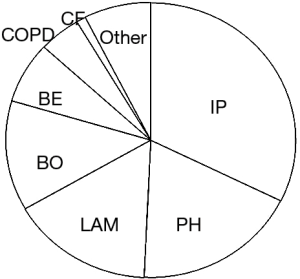
Regarding recipient’s diagnoses, interstitial lung pneumonia, pulmonary hypertension, lymphangioleiomyomatosis, and bronchiolitis obliterans were four major indications (Figure 3). Indications for lung transplant are quite distinct in Japan where cystic fibrosis is a rare disease and emphysema is not a common indication due to upper age limit. Majority patients with bronchiolitis obliterans suffered from chronic progressive pulmonary complications after hematopoietic stem cell transplantation (6,7). These patients are commonly believed to be high-risk candidates for lung transplantation because long term pre-transplant intake of immunosuppressant drugs often causes organ dysfunction of other organs and increases the risk of various infections.
Strategies for organ shortage
As the waiting list for lung transplantation continues to grow, use of DCD has recently begun to expand. The Maastricht workshop identified categories of donors that could be considered for DCD. Category 1: dead on arrival. Category 2: unsuccessful resuscitation, are uncontrolled donors. Spanish group utilized Category 1 donors and reported acceptable mid- and long-term survival, however, the higher rated of primary graft dysfunction and its impact on early mortality were also reported (8). Category 3: includes donors awaiting cardiac arrest, and category 4 involves cardiac arrest in a brain dead donor. Maastricht category three donors were increasingly used for lung transplantation in Europe, Australia and North America (9-13). Withdrawal of life-supporting therapy is planned in advance in the setting of an intensive care unit or operating room. After confirming circulatory arrest for the legally determined minutes, the lungs are extracted and used for transplantation. The results after category three were reported to be at least equivalent to that after brain dead donation. However, controlled death is not permitted in Japan making it difficult to accept this strategy.
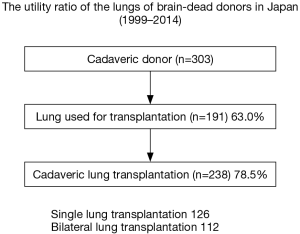
Use of marginal donors is one of the strategies for organ shortage. For example, we successfully performed bilateral lung transplantation from a donor with ruptured right main bronchus by a trauma (14). A medical consult system has been established in Japan to maintain various organ conditions suitable for the subsequent donation. The utility ratio of the lungs of brain-dead donors in Japan (Figure 4) is indeed over 60% and it is much higher than that reported in the USA (approximately 20%). Bilateral lung transplantation has been increasingly performed in most of the centers knowing that its long term survival is better than that of single lung transplantation (1). However, single lung transplantation has been chosen more often than bilateral lung transplantation in Japan to maximize the number of transplantation by sharing the scarce donor (5,15). As a result, donor/recipient ratio was approaching 80%.
LDLLT has been performed as a life-saving procedure for critically ill patients who are unlikely to survive the long wait for cadaveric lungs (3,4,16,17). Although LDLLT was initially performed in the USA (18), its use has decreased there because of the recent change by the Organ Procurement and Transplantation Network to an urgency/benefit allocation system for cadaveric donor lungs. For the past several years, reports on LDLLT almost exclusively have been from Japan, where the average waiting time for a cadaveric lung is still more than 800 days. Because only two lobes are implanted, appropriate size matching between the donor and recipient is important in LDLLT. It is often inevitable that small grafts are implanted. Excessively small grafts may cause high pulmonary artery pressure, resulting in lung edema. We have developed lobar sparing transplantation (19) and right to left inverted transplantation (20) for undersize graft. On the other hand, the adult lower lobe might be too big for small children. The use of oversized grafts could cause high airway resistance, atelectasis and hemodynamic instability by the time of chest closure. To overcome these problems, we have developed several techniques including single lobe transplantation with or without contralateral pneumonectomy (21,22), delayed chest closure (23), and downsizing the graft (24).
Comparison between CLT and LDLLT
Advantages and disadvantages of LDLLT compared to CLT are summarized in Table 1. In general, the ischemic time for LDLLT is much shorter than CLT. Although only two lobes are transplanted, LDLLT seems to be associated with less frequent primary graft failure. We believe that using a “small but perfect graft” is a great advantage in LDLLT.
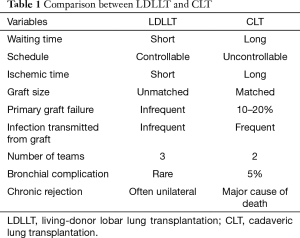
Full table
Experienced centers have recently reported the incidence of bronchial complications in CLT to be about 5%. Contraindications to CLT include current high-dose systemic corticosteroid therapy because it may increase airway complications, although low-dose pre-transplantation corticosteroid therapy is acceptable. We have accepted high-dose systemic corticosteroid therapy in LDLLT. Excellent bronchial healing was observed in spite of high-dose steroid use (25). Various factors, such as short donor bronchial length, high blood flow in the small grafts implanted, well preserved lung parenchyma with short ischemic time, may contribute to better oxygen supply to the donor bronchus resulting in excellent bronchial healing in LDLLT.
Chronic allograft dysfunction (CLAD) including bronchiolitis obliterans syndrome (BOS) has been the major obstacle after CLT. Theoretically, human leukocyte antigen (HLA) similarity in cases of blood related donation may be beneficial, but the role of HLA mismatches may be more complicated when two different donor lobes are implanted into one recipient in bilateral LDLLT. Transplanting two lobes obtained from two different donors appears to be beneficial in the long term because the contralateral unaffected lung may function as a reservoir in case of unilateral BOS (26). The question remains whether patients receiving LDLLT will have less CLAD than those receiving CLT.
Results after lung transplantation
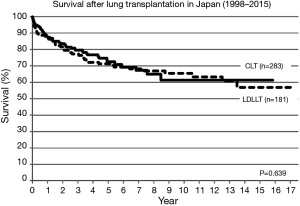
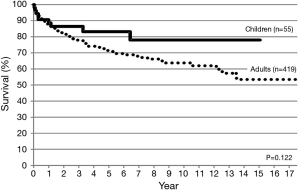
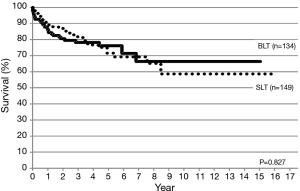
Between 1998 and 2015, lung transplantation has been performed in 464 patients (55 children, 419 adults) at nine lung transplant centers in Japan. CLT was performed in 283 patients (61%) and LDLLT was performed in 181 patients (39%). The 5-year survival was 72.3% and 71.6%, respectively (Figure 5). Among 55 pediatric patients, only 7 children (12.7%) received CLT and 48 children (87.3%) received LDLLT. Although the number of pediatric patients was small, pediatric lung transplant recipients showed a trend toward better long-term survival (Figure 6). Among 283 patients receiving CLT, single lung transplantation was performed in 149 patients (52.7%) and bilateral lung transplantation was performed in 134 patients (47.3%). The survival was similar between the two procedures (Figure 7).
Summary
Lung transplantation in Japan has grown significantly with excellent results but the shortage of cadaveric lung donor remains to be an important unsolved problem. The average waiting time is more than 800 days and the mortality rate on the waiting list is nearly 50%. Use of marginal donor, medical consults system, and single lung transplantation result in high utilization ratio of the lungs of brain-dead donors. Despite the aggressive use of marginal lungs and single lung transplantation by Japanese lung transplant centers, the overall survival of recipients in Japan (5-year survival, approximately 70%) is generally better than that reported by the International Society for Heart and Lung Transplantation registry (5-year survival, approximately 50%). LDLLT is often the only realistic option for very ill patients, especially for children. Although LDLLT patients are in worse preoperative condition than CLT patients, LDLLT patients demonstrates survival rates similar to CLT patients (17).
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirty-second official adult lung and heart-lung transplantation report--2015; focus theme: early graft failure. J Heart Lung Transplant 2015;34:1264-77. [Crossref] [PubMed]
- Date H, Yamashita M, Nagahiro I, et al. Living-donor lobar lung transplantation for primary ciliary dyskinesia. Ann Thorac Surg 2001;71:2008-9. [Crossref] [PubMed]
- Date H, Aoe M, Nagahiro I, et al. Living-donor lobar lung transplantation for various lung diseases. J Thorac Cardiovasc Surg 2003;126:476-81. [Crossref] [PubMed]
- Date H, Aoe M, Sano Y, et al. Improved survival after living-donor lobar lung transplantation. J Thorac Cardiovasc Surg 2004;128:933-40. [Crossref] [PubMed]
- Sato M, Okada Y, Oto T, et al. Registry of the Japanese Society of Lung and Heart-Lung Transplantation: official Japanese lung transplantation report, 2014. Gen Thorac Cardiovasc Surg 2014;62:594-601. [Crossref] [PubMed]
- Yamane M, Sano Y, Toyooka S, et al. Living-donor lobar lung transplantation for pulmonary complications after hematopoietic stem cell transplantation. Transplantation 2008;86:1767-70. [Crossref] [PubMed]
- Chen F, Yamane M, Inoue M, et al. Less maintenance immunosuppression in lung transplantation following hematopoietic stem cell transplantation from the same living donor. Am J Transplant 2011;11:1509-16. [Crossref] [PubMed]
- Gomez-de-Antonio D, Campo-Cañaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant 2012;31:349-53. [Crossref] [PubMed]
- Mason DP, Thuita L, Alster JM, et al. Should lung transplantation be performed using donation after cardiac death? The United States experience. J Thorac Cardiovasc Surg 2008;136:1061-6. [Crossref] [PubMed]
- Snell GI, Levvey BJ, Oto T, et al. Early lung transplantation success utilizing controlled donation after cardiac death donors. Am J Transplant 2008;8:1282-9. [Crossref] [PubMed]
- Cypel M, Levvey B, Van Raemdonck D, et al. International society for heart and lung transplantation donation after circulatory death registry report. J Heart Lung Transplant 2015;34:1278-82. [Crossref] [PubMed]
- Krutsinger D, Reed RM, Blevins A, et al. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant 2015;34:675-84. [Crossref] [PubMed]
- Sabashnikov A, Patil NP, Popov AF. Long-term results after lung transplantation using organs from circulatory death donors: a propensity score-matched analysis†. Eur J Cardiothorac Surg 2016;49:46-53. [Crossref] [PubMed]
- Miyamoto E, Sato M, Yamada T, et al. Successful bilateral lung transplantation from a deceased donor with a ruptured main bronchus. Interact Cardiovasc Thorac Surg 2015;21:396-8. [Crossref] [PubMed]
- Miyoshi R, Chen-Yoshikawa TF, Hijiya K, et al. Significance of single lung transplantation in the current situation of severe donor shortage in Japan. Gen Thorac Cardiovasc Surg 2016;64:93-7. [Crossref] [PubMed]
- Date H. Update on living-donor lobar lung transplantation. Curr Opin Organ Transplant 2011;16:453-7. [Crossref] [PubMed]
- Date H, Sato M, Aoyama A, et al. Living-donor lobar lung transplantation provides similar survival to cadaveric lung transplantation even for very ill patients†. Eur J Cardiothorac Surg 2015;47:967-72; discussion 972-3. [Crossref] [PubMed]
- Starnes VA, Bowdish ME, Woo MS, et al. A decade of living lobar lung transplantation: recipient outcomes. J Thorac Cardiovasc Surg 2004;127:114-22. [Crossref] [PubMed]
- Aoyama A, Chen F, Minakata K, et al. Sparing native upper lobes in living-donor lobar lung transplantation: five cases from a single center. Am J Transplant 2015;15:3202-7. [Crossref] [PubMed]
- Chen F, Miyamoto E, Takemoto M, et al. Right and left inverted lobar lung transplantation. Am J Transplant 2015;15:1716-21. [Crossref] [PubMed]
- Sonobe M, Bando T, Kusuki S, et al. Living-donor, single-lobe lung transplantation and simultaneous contralateral pneumonectomy in a child. J Heart Lung Transplant 2011;30:471-4. [Crossref] [PubMed]
- Date H, Shiraishi T, Sugimoto S, et al. Outcome of living-donor lobar lung transplantation using a single donor. J Thorac Cardiovasc Surg 2012;144:710-5. [Crossref] [PubMed]
- Chen F, Matsukawa S, Ishii H, et al. Delayed chest closure assessed by transesophageal echocardiogram in single-lobe lung transplantation. Ann Thorac Surg 2011;92:2254-7. [Crossref] [PubMed]
- Chen F, Fujinaga T, Shoji T, et al. Perioperative assessment of oversized lobar graft downsizing in living-donor lobar lung transplantation using three-dimensional computed tomographic volumetry. Transpl Int 2010;23:e41-4. [PubMed]
- Toyooka S, Yamane M, Oto T, et al. Bronchial healing after living-donor lobar lung transplantation. Surg Today 2009;39:938-43. [Crossref] [PubMed]
- Miyamoto E, Chen F, Aoyama A, et al. Unilateral chronic lung allograft dysfunction is a characteristic of bilateral living-donor lobar lung transplantation. Eur J Cardiothorac Surg 2015;48:463-9. [Crossref] [PubMed]


