Oxygen desaturation during a 6-minute walk test as a predictor of maximal exercise-induced gas exchange abnormalities in sarcoidosis
Introduction
Sarcoidosis is a systemic granulomatous disorder preferentially involving the lung. Pulmonary sarcoidosis is usually benign. Although gas exchange measurements in interstitial lung disease usually include the diffusing capacity of the lung for carbon monoxide (DLco), it is unclear whether estimates of gas exchange might be improved by measuring oxygen desaturation (∆SpO2) during a 6-minute walk test (6MWT) and/or the alveolar-to-arterial oxygen pressure difference measured at peak exercise (peak AaDO2) during a cardiopulmonary exercise test (CPET) (1-5). On the one hand, peak AaDO2 is more sensitive than DLco (3,6,7), but CPET requires time and equipment and is challenging for sarcoidosis patients (8), limiting its use for systematic initial evaluation and for routine monitoring (9). On the other hand, ∆SpO2 during a 6MWT (∆SpO2-6MWT) is a measure of gas exchange at exercise and is a simple, reproducible, and well-tolerated exam (10), which are advantages for sarcoidosis evaluation (11). Measurement of ∆SpO2-6MWT as performed in the present study has recently been added to the international guidelines for walking tests (12). Although this test has not been investigated extensively, it is reproducible (13) and correlates with symptom intensity (14), results of pulmonary function testing (15), changes on computed tomography (CT) scans (16), and prognosis (17-19) in interstitial lung diseases. However, the correlation between ∆SpO2-6MWT and peak AaDO2 and, in particular, the ability of ∆SpO2-6MWT to predict peak AaDO2, are unknown. In the present study, we investigated in a large population of sarcoidosis patients the relationship between DLco and two other indicators of gas exchange; namely, ∆SpO2-6MWT and peak AaDO2, using correlation and inter-test agreement tests and evaluated the ability of ∆SpO2-6MWT and DLco to predict gas exchange impairment during exercise in sarcoidosis.
Methods
Subjects and study design
We conducted a monocentric retrospective study of 130 subjects. All sarcoidosis patients referred to our tertiary respiratory care clinic over a period of 6 years were considered for inclusion. Subjects were included if the diagnosis of sarcoidosis was histologically proved and if they had performed a 6MWT and a CPET with blood gas measurement at peak exercise on the same day. Exercise tests were performed either at the time of diagnosis or during the course of the disease. If a patient had performed multiple tests, the first complete evaluation was selected for analysis. Demographic, clinical, radiological, and functional data, including pulmonary function tests, 6MWT, and CPET, were recorded. The relationships between DLco, ∆SpO2-6MWT, and peak AaDO2 were analyzed.
Sarcoidosis classification
Pulmonary sarcoidosis was classified into five stages according to the ERS/ATS statement on sarcoidosis as follows: stage 0, no visible intrathoracic findings; stage I, bilateral hilar adenopathy; stage II, bilateral hilar adenopathy and parenchymal infiltration; stage III, parenchymal infiltration without hilar adenopathy; and stage IV, evidence of pulmonary fibrosis with cicatricial changes (9).
Pulmonary function tests
Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and total lung capacity (TLC) were measured by spirometry and plethysmography (Jaeger-Masterlab®). Single-breath DLco was measured. The DLco value was adjusted for hemoglobin by applying Cotes’ equation: adjusted DLco = [DLco × (10.2 + hemoglobin)/(1.7 × hemoglobin)]. Predicted values for lung volumes and DLco were based on the ERS statement (20,21). DLco was considered normal if it was above the lower limit of normal (LLN), defined as the 5th percentile (22). Resting arterial blood gas tensions were measured on room air. Normal values for resting partial pressure of oxygen (PaO2) and AaDO2 were derived from the literature (23,24).
6MWT
The 6MWT was performed in accordance with international recommendations (10). Peripheral capillary oxygen saturation (SpO2) was monitored continuously using a handheld oximeter (Nellcor OxiMax® N-65). ∆SpO2-6MWT was defined as the difference between the resting and nadir SpO2. A desaturation ≥4% was considered significant (25).
CPET
CPET was performed to investigate dyspnea in 85 patients (66%) and other symptoms, including fatigue and exercise intolerance, in 45 patients (34%). Subjects completed a triangular exercise test on an ergometric bicycle (Ergoline-Ergometrics 800®) using a standardized protocol, as detailed previously (26). Expired gases were extracted from each respiratory cycle with an Ergocard®. Heart rate and SpO2 were monitored continuously using a 12-lead electrocardiogram and a pulse oximeter (Nellcor N-395), respectively. Arterial blood sampling was performed on room air at rest and at peak exercise. AaDO2 was calculated from PAO2, the ‘ideal’ compartment alveolar PO2 determined from the alveolar gas equation [PAO2 = PiO2 − (PaCO2/RER)] and PaO2. Based on the age of the population, peak AaDO2 above 30 mmHg was considered increased (27).
Statistics
Statistical analyses were performed using SPSS software (IBM SPSS Statistics 21). Normality of data distribution was assessed using the Shapiro-Wilk test. Quantitative data are presented as the median and interquartile range indicated in brackets. Qualitative data are presented as frequencies and percentages. Dependent variables (results of pulmonary function tests, blood gas measures, and 6MWT) were compared between disease stages using one-way ANOVA when data were normally distributed in all groups and using the Kruskal-Wallis test when at least one dataset was not normally distributed. Post hoc pairwise comparisons were performed using significance levels adjusted for multiplicity. Correlations between the quantitative variables DLco, ∆SpO2-6MWT, and peak AaDO2 in the whole population were evaluated using a bivariate Pearson test. Inter-test reliability, predictive values, and ROC curves were derived using DLco, ∆SpO2-6MWT, and peak AaDO2 as binary qualitative variables (normal vs. abnormal) according to the thresholds stated above (DLco < LLN, ∆SpO2-6MWT ≥4%, peak AaDO2 >30 mmHg). Concordance between DLco, ∆SpO2-6MWT, and peak AaDO2 was evaluated using the Kappa coefficient test with 95% confidence intervals. Negative and positive predictive values (NPV and PPV, respectively) were calculated, first according to the previously defined threshold and second according to thresholds characteristic of severe hypoxemia (nadir SpO2 <90% during the 6MWT and PaO2 <60 mmHg at peak exercise during CPET). ROC curves were calculated to determine whether more relevant thresholds could be identified. A correction for multiplicity was applied to descriptive results, correlation tests, and Kappa coefficients using the Benjamini-Hochberg procedure. A P value <0.05 was considered significant.
Results
Subjects
One hundred and thirty subjects were included. The population consisted of 52 women (40%) and 78 men (60%), of whom 114 were Caucasians (87%) and 16 were Africans (13%). The mean ± SD age was 49±11 years (range, 26–78 years) and the mean body mass index was 27 kg·m−2. Sixty percent of the population had no smoking history, 15% were active smokers, and 25% were ex-smokers. Pulmonary sarcoidosis was classified as stage I in 11 subjects (8.5%), stage II in 69 subjects (53%), stage III in 11 subjects (8.5%), and stage IV in 39 subjects (30%). At the time of functional exercise testing, the proportion of subjects receiving corticosteroids was 10% of stage I subjects, 44% of stage II subjects, 36% of stage III subjects, and 76% of stage IV subjects. The proportion of steroid treatment was significant differences between all groups.
Physiological evaluation
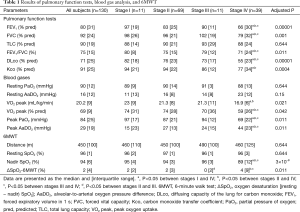
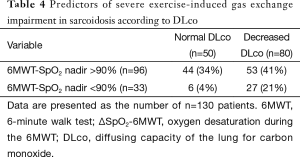
Pulmonary function tests, AaDO2 at rest and peak exercise, and 6MWT results are detailed in Table 1. In summary, the stage IV sarcoidosis group had lower FEV1, FVC, FEV1/FVC, DLco, peak oxygen uptake (VO2 peak), and peak PaO2 and higher AaDO2 than the stage I, II, and III groups. There were no significant differences in these variables between stage I, II, and III groups. ΔSpO2-6MWT was higher in the stage IV group than in stage II and III groups, and higher in the stage II group than in the stage III group. The prevalence of obstructive and restrictive ventilatory defects was similar to those previously published (1,7,28), and was 23% for airway obstruction (FEV1/FVC < LLN), 21% for airway restriction (TLC <80% of predicted value), and 61.5% for decreased DLco (DLco < LLN). Resting PaO2 and AaDO2 were considered abnormal according to literature normal values (23,24) in 40% and 30% of cases, respectively. In most of these abnormal cases, the parameters were not markedly altered (25th percentile PaO2 of 84 mmHg and 75th percentile AaDO2 of 23 mmHg). Twenty-six subjects (20%) showed severe desaturation (nadir SpO2 <90%) during the 6MWT, and 16 subjects (12%) showed severe hypoxemia (PaO2 <60 mmHg) at peak exercise during CPET. Peak AaDO2 and ΔSpO2-6MWT were increased in 49% and 27% of cases, respectively.
Full table
Correlations
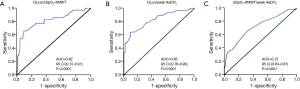
ΔSpO2-6MWT and peak AaDO2 were both significantly correlated with DLco with intermediate correlation coefficients (Figure 1). ΔSpO2-6MWT was also significantly correlated with peak AaDO2 with a moderate correlation coefficient (Figure 1).
Inter-test agreement analysis
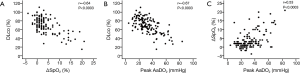
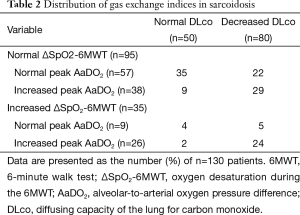
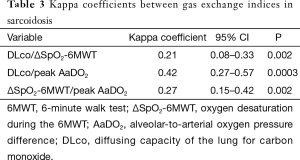
To assess the concordance between DLco, ΔSpO2-6MWT, and peak AaDO2 as predictors of gas exchange impairment, the variables were expressed as “normal” versus “abnormal” according to previously established thresholds. Gas exchange indices were all normal in 35 subjects (27%) and all altered in 24 subjects (18%), giving an overall concordance of 45% (Table 2). In 46 cases (35%), ΔSpO2-6MWT and peak AaDO2 were discordant. Kappa coefficients between 0.21 and 0.42 confirmed modest inter-test reliability (Table 3).
Full table
Full table
Predictive values
We evaluated the ability of DLco to predict ∆SpO2-6MWT and peak AaDO2 and the ability of ∆SpO2-6MWT to predict peak AaDO2 using transformed binary variables. DLco was a poor predictor of ∆SpO2-6MWT (PPV 36% and NPV 88%) and of peak AaDO2 (PPV 66% and NPV 78%). Importantly, normal DLco was a good predictor of the absence of severe desaturation during the 6MWT and at peak exercise during CPET (NPV 94% and 100%, respectively). In contrast, decreased DLco did not predict severe desaturation during the 6MWT or at peak exercise during CPET (PPV 27% and 19%, respectively). ∆SpO2-6MWT predicted peak AaDO2 with low NPV (60%) and PPV (74%) (Tables 4,5). ROC curve analyses did not identify more relevant thresholds than those previously defined (Figure 2).
Full table

Full table
Discussion
In the present study of a large population of patients with sarcoidosis, we found that DLco, ∆SpO2-6MWT, and peak AaDO2 showed discordant results. In more than 50% of cases, one parameter among DLco, ∆SpO2-6MWT, and peak AaDO2 conflicted with another parameter. These results were corroborated by the low to intermediate correlation coefficients, low Kappa coefficients, and low predictive values. However, ΔSpO2-6MWT and peak AaDO2 were consistent measures of gas exchange since they both correlated with DLco and correlated with each other. DLco was a poor predictor of exercise-induced gas exchange impairment evaluated by ∆SpO2-6MWT or peak AaDO2.
Study limitations
The main limitation of the study concerns the definition of significance for peak AaDO2 and ∆SpO2-6MWT. We selected a threshold of 30 mmHg for peak AaDO2 based on international recommendations proposing a range of 30–35 mmHg (29) and on the average age of our study population (27). Although this choice might have overestimated the proportion of subjects with increased peak AaDO2, it is unlikely to have influenced the finding of discordance between gas exchange indices because only 14 subjects (11%) had a peak AaDO2 between 30 and 35 mmHg, and the distribution of these patients between disease groups was not significantly different (data not shown). For ∆SpO2-6MWT, we chose a threshold desaturation of 4% based on published data (25). This threshold cannot be implicated in the discrepancy between gas exchange indices because the ROC curves failed to identify a better threshold to predict peak AaDO2.
Physiological interpretation and implications for clinical practice
There were no differences in the distance walked during the 6MWT among the stage I–IV groups. This is in accordance with a previous study reporting a lack of correlation between sarcoidosis stages and the exercise tolerance or 6MWT distance (26,30). We hypothesize that the main exercise-limiting factor in sarcoidosis is not the physiological response to exercise but the symptoms, especially fatigue; however, this remains to be tested.
Normal DLco and impaired gas exchange during exercise
Thirty percent of subjects with normal DLco showed impaired exercise-related gas exchange, as indicated by increased ∆SpO2-6MWT and/or peak AaDO2. In chronic respiratory diseases of moderate severity, gas exchange efficiency may be conserved at rest but become insufficient during exercise. Sarcoidosis patients fail to increase the carbon monoxide transfer coefficient during exercise (31) due to bronchiolar obstruction, increased alveolocapillary membrane thickness, and vascular involvement (32). This result confirms that exercise tests improve the detection of gas exchange impairment in sarcoidosis, as previously reported for peak AaDO2 (7). The present study shows that ∆SpO2-6MWT is also a more sensitive index than DLco to assess gas exchange impairment in sarcoidosis. For clinical practice, gas exchange evaluation during the 6MWT and CPET might be helpful to understand symptoms unexplained by resting exams and to appreciate better the disease severity.
Discordance between exercise tests
In this study, 30% of subjects showed normal ∆SpO2-6MWT but increased peak AaDO2. This could be related in part to the lower workload elicited by the 6MWT (28) and to the lower sensitivity of SpO2 compared with PaO2 changes. Conversely, the 6MWT could reveal exercise-induced gas exchange impairment in patients with normal peak AaDO2, which might be particularly helpful in the case of patients with unexplained symptoms and normal peak AaDO2. Such discordance between exercise tests has previously been reported in idiopathic pulmonary fibrosis (33) and chronic obstructive pulmonary disease (34), but not in sarcoidosis. This could be related to the tests employed, since alveolar ventilation is lower and anaerobic metabolism is delayed during walking compared with cycling (35,36). Finally, the 6MWT and CPET provide complementary information on gas exchange adjustment during exercise. Interestingly, 17% of our patients presented with decreased DLco but normal ∆SpO2-6MWT and peak AaDO2. On explanation may be that normalization of gas exchange during exercise in sarcoidosis patients most likely results from improvement of ventilation/perfusion mismatch, although this has not been extensively investigated. Indeed, exercise may preferentially recruit the healthiest zones of the lung during exercise, as they are more compliant than inflamed areas.
Conclusions
In the present study, DLco, ∆SpO2-6MWT, and peak AaDO2 were independent measures of gas exchange in a large population of sarcoidosis patients. Neither ∆SpO2-6MWT nor DLco was a good predictor of increased peak AaDO2. In contrast, the main implication for clinical practice is that normal DLco is a good predictor of the absence of severe gas exchange impairment. ∆SpO2-6MWT could represent a new index that provides distinct information of relevance for sarcoidosis evaluation and management, but this needs to be investigated by prospective and longitudinal studies.
Acknowledgements
The authors wish to thank Anne M. O’Rourke for editing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors disclose professional relationships with companies or manufacturers that will benefit from the results of the present study.
Ethical Statement: The study was approved by the Institutional Review Board of the French Learned Society for Respiratory Medicine (No. CEPRO 2011-039) and written informed consent was obtained from all patients.
References
- Barros WG, Neder JA, Pereira CA, et al. Clinical, radiographic and functional predictors of pulmonary gas exchange impairment at moderate exercise in patients with sarcoidosis. Respiration 2004;71:367-73. [Crossref] [PubMed]
- Drent M, De Vries J, Lenters M, et al. Sarcoidosis: assessment of disease severity using HRCT. Eur Radiol 2003;13:2462-71. [Crossref] [PubMed]
- Lamberto C, Nunes H, Le Toumelin P, et al. Membrane and capillary blood components of diffusion capacity of the lung for carbon monoxide in pulmonary sarcoidosis: relation to exercise gas exchange. Chest 2004;125:2061-8. [Crossref] [PubMed]
- Medinger AE, Khouri S, Rohatgi PK. Sarcoidosis: the value of exercise testing. Chest 2001;120:93-101. [Crossref] [PubMed]
- Miller A, Brown LK, Sloane MF, et al. Cardiorespiratory responses to incremental exercise in sarcoidosis patients with normal spirometry. Chest 1995;107:323-9. [Crossref] [PubMed]
- Kollert F, Geck B, Suchy R, et al. The impact of gas exchange measurement during exercise in pulmonary sarcoidosis. Respir Med 2011;105:122-9. [Crossref] [PubMed]
- Marcellis RG, Lenssen AF, de Vries GJ, et al. Is there an added value of cardiopulmonary exercise testing in sarcoidosis patients? Lung 2013;191:43-52. [Crossref] [PubMed]
- Braam AW, de Haan SN, Vorselaars AD, et al. Influence of repeated maximal exercise testing on biomarkers and fatigue in sarcoidosis. Brain Behav Immun 2013;33:57-64. [Crossref] [PubMed]
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160:736-55. [PubMed]
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-7. [Crossref] [PubMed]
- Baughman RP, Lower EE. Six-minute walk test in managing and monitoring sarcoidosis patients. Curr Opin Pulm Med 2007;13:439-44. [Crossref] [PubMed]
- Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014;44:1428-46. [Crossref] [PubMed]
- Wilsher M, Good N, Hopkins R, et al. The six-minute walk test using forehead oximetry is reliable in the assessment of scleroderma lung disease. Respirology 2012;17:647-52. [Crossref] [PubMed]
- Manali ED, Lyberopoulos P, Triantafillidou C, et al. MRC chronic Dyspnea Scale: Relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study. BMC Pulm Med 2010;10:32. [Crossref] [PubMed]
- Chetta A, Aiello M, Foresi A, et al. Relationship between outcome measures of six-minute walk test and baseline lung function in patients with interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis 2001;18:170-5. [PubMed]
- Baldi BG, Araujo MS, Freitas CS, et al. Evaluation of the extent of pulmonary cysts and their association with functional variables and serum markers in lymphangioleiomyomatosis (LAM). Lung 2014;192:967-74. [Crossref] [PubMed]
- Eaton T, Young P, Milne D, et al. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 2005;171:1150-7. [Crossref] [PubMed]
- Hallstrand TS, Boitano LJ, Johnson WC, et al. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J 2005;25:96-103. [Crossref] [PubMed]
- Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003;168:1084-90. [Crossref] [PubMed]
- Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:1-100. [PubMed]
- Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J 1995;8:492-506. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [Crossref] [PubMed]
- Kanber GJ, King FW, Eshchar YR, et al. The alveolar-arterial oxygen gradient in young and elderly men during air and oxygen breathing. Am Rev Respir Dis 1968;97:376-81. [PubMed]
- Sorbini CA, Grassi V, Solinas E, et al. Arterial oxygen tension in relation to age in healthy subjects. Respiration 1968;25:3-13. [Crossref] [PubMed]
- Prefaut C, Durand F, Mucci P, et al. Exercise-induced arterial hypoxaemia in athletes: a review. Sports Med 2000;30:47-61. [Crossref] [PubMed]
- Wallaert B, Talleu C, Wemeau-Stervinou L, et al. Reduction of maximal oxygen uptake in sarcoidosis: relationship with disease severity. Respiration 2011;82:501-8. [Crossref] [PubMed]
- Aguilaniu B, Maitre J, Diab S, et al. Detection of disturbances in pulmonary gas exchanges during exercise from arterialized earlobe PO2. Respir Physiol Neurobiol 2011;177:30-5. [Crossref] [PubMed]
- Boros PW, Enright PL, Quanjer PH, et al. Impaired lung compliance and DL,CO but no restrictive ventilatory defect in sarcoidosis. Eur Respir J 2010;36:1315-22. [Crossref] [PubMed]
- Ross RM. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:1451; author reply 1451. [Crossref] [PubMed]
- Alhamad EH. The six-minute walk test in patients with pulmonary sarcoidosis. Ann Thorac Med 2009;4:60-4. [Crossref] [PubMed]
- Young RC Jr, Fields HP, Scott JM, et al. Lung diffusing capacity response to exercise in sarcoidosis. A physiologic study. J Natl Med Assoc 1969;61:508-13. [PubMed]
- Polychronopoulos VS, Prakash UB. Airway involvement in sarcoidosis. Chest 2009;136:1371-80. [Crossref] [PubMed]
- Wallaert B, Wemeau-Stervinou L, Salleron J, et al. Do we need exercise tests to detect gas exchange impairment in fibrotic idiopathic interstitial pneumonias? Pulm Med 2012;2012:657180.
- Poulain M, Durand F, Palomba B, et al. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003;123:1401-7. [Crossref] [PubMed]
- Cockcroft A, Beaumont A, Adams L, et al. Arterial oxygen desaturation during treadmill and bicycle exercise in patients with chronic obstructive airways disease. Clin Sci (Lond) 1985;68:327-32. [Crossref] [PubMed]
- Mahler DA, Gifford AH, Waterman LA, et al. Mechanism of greater oxygen desaturation during walking compared with cycling in patients with COPD. Chest 2011;140:351-8. [Crossref] [PubMed]


