The role of surgical intervention in lung cancer with carcinomatous pleuritis
Introduction
Non-small cell lung cancer (NSCLC) associated with carcinomatous pleuritis, which includes malignant pleural effusion (MPE) and/or malignant pleural nodules (MPNs), is generally considered to be incurable, and in the latest 7th edition of the tumor-node-metastasis (TNM) classification these cases have been classified as stage IV disease (1). The International Association for the Study of Lung Cancer (IASLC) reviewed the former TMN staging for lung cancer and recommended several revisions in 2007 (2,3). One of the most significant revisions was to classify carcinomatous pleural disease, either with MPE or MPNs without evidence of other metastatic disease, under the M1a category, making it stage IV disease. IASLC also reported the 1- and 5-year survival rates of patients with carcinomatous pleuritis were 36% and 2%, respectively, using their large globally collected cohort. This is a worse outcome than for other T4M0 cases, but is better than that of patients with distant metastases, where the median survival is only 4 to 7 months.
NSCLC patients only found to have carcinomatous pleuritis at thoracotomy are also classified as having stage IV disease, even though they are expected to have minimal disease. In clinical practice, surgeons sometimes encounter such patients with ‘occult’ carcinomatous pleuritis; however, the standard treatment for such patients is not established in any clinical guidelines. Thus, various treatments such as the best supportive care, systemic chemotherapies using cytotoxic agents or molecular targeted drugs, or surgical interventions including extrapleural pneumonectomy (EPP), have been employed in Japan (4-11).
This review article is intended to provide an overview of our current understanding of carcinomatous pleuritis of NSCLC and to discuss the clinical implications of surgical interventions for patients with carcinomatous pleuritis.
Patients with carcinomatous pleuritis detected at thoracotomy
Patients with carcinomatous pleuritis are acknowledged to be very diverse; some have minimal amounts of MPE, which is first detected at thoracotomy; some have numerous MPNs without any effusion; and others have massive amounts of MPE and MPNs with symptoms. For patients with massive MPE and/or MPNs, which is apparent by radiological evaluation, systemic chemotherapy is the gold standard of treatment, the same as for other types of stage IV disease. However, the survival outcome of these patients is thought to be poor. In addition, whether MPE and MPN represent the same disease condition is unclear. Several studies have reported that there is no significant difference in survival outcomes between patients with MPE or MPN (4,8), but Fukuse et al. demonstrated that patients with MPE had a better prognosis than those with MPN (5).
The population of patients with carcinomatous pleuritis first detected at thoracotomy ranges from 1.5% to 4.5% for all surgical cases (4,5,8,12,13) (Table 1). Ichinose et al. reported the first series of 284 patients (3.2%) found to have carcinomatous pleuritis at thoracotomy among 8,813 patients collected by the Japan Clinical Oncology Group (JCOG) (4). The Japanese Joint Committee of Lung Cancer Registry reported that among the 11,420 registered NSCLC patients who underwent surgical intervention in 2004, 329 patients had carcinomatous pleuritis (2.9%) (14). The population has not changed during the last decade.
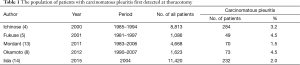
Full table
Iida et al. reported an elevation of preoperative serum tumor markers, which include carcinoembryonic antigen, squamous cell carcinoma-related antigen, cytokeratin 19 fragment, Sialyl Lewis X, neuron-specific enolase, and progastrin-releasing peptide; nonsquamous cell carcinoma histology; larger tumor size; and lymph node involvement were significantly associated with a higher incidence of carcinomatous pleuritis (14).
Surgical intervention for carcinomatous pleuritis
The prognostic outcomes of patients with carcinomatous pleuritis have also been recognized to be variable, because of the heterogeneity in the extent of disease and the amount of effusion. Pulmonary resections, including partial resection, segmentectomy, lobectomy, and pneumonectomy, are generally contraindicated for patients with carcinomatous pleuritis. However, several investigators have reported that the postoperative prognosis of patients with carcinomatous pleuritis discovered at thoracotomy was relatively favorable (4,6,8,13,15) (Table 2).

Full table
Ichinose et al. reported the outcomes of 193 patients with carcinomatous pleuritis who underwent pulmonary resections, including 29 pneumonectomies, using the cohort of the JCOG described above (4). Among them, no gross residual tumor apart from the carcinomatous pleuritis remained in 155 (69%) patients. The 3- and 5-year survival rates of the 193 patients who underwent resection were 28.8% and 14.9%, respectively. They also analyzed the prognosis of 100 patients with minimal disease carcinomatous pleuritis. Minimal disease consisted of three conditions: no MPE and a small number of MPNs, MPE less than 300 mL and no MPNs, and MPE less than 300 mL and a small number of MPNs. The 3- and 5-year survival rates were 31.8% and 22.8%, respectively. The authors commented that the outcomes of resected NSCLC patients with minimal disease carcinomatous pleuritis were unexpectedly good.
Fukuse et al. evaluated 49 Japanese patients with lung cancer who were first diagnosed with MPE and/or MPN at thoracotomy (5). Partial resection was performed in seven patients, lobectomy in 27, and EPP in five. Radical dissection of the mediastinal and hilar lymph nodes was performed in the 32 patients who underwent lobectomy or EPP. The median survival times of the partial resection and lobectomy patients were 23.2 and 37.9 months, respectively.
In France, Mordant et al. investigated 32 patients with unexpected carcinomatous pleuritis at thoracotomy who underwent attempted curative pulmonary resection (13). A total of nine pneumonectomies and 23 lobectomies, along with mediastinal lymph node dissection and surgical resection of MPNs, were performed. The 5-year survival rate was 16% after resection, and 21% if the resection was a lobectomy.
Using the Japanese Joint Committee of Lung Cancer Registry, Iida et al. also reported the survival outcomes of patients with carcinomatous pleuritis (14). The median survival time and 5-year survival rate of 313 patients without other metastatic disease were 34.0 months and 29.3%, respectively. Primary tumor resection was performed in 256 (81.8%) patients, and macroscopic complete resection was achieved in 152 (48.6%) patients, with 5-year survival rates of 33.1% and 37.1%, respectively. The authors concluded achieving macroscopic complete resection was an independent prognostic factor.
Previous reports showed relatively higher 5-year survival rates of 15% to 37% for surgically treated cases while 5-year survival of all patients with M1a disease was estimated at 2% by the IASLC (2). Thus, surgical intervention, including major pulmonary resections, might contribute to better survival of patients with minimal disease carcinomatous pleuritis.
EPP for carcinomatous pleuritis
EPP is an en bloc resection of the lung along with the parietal and visceral pleurae and also usually the ipsilateral diaphragm and pericardium. EPP is commonly employed in the treatment of malignant pleural mesothelioma. A few authors have reported that they have successfully performed EPP in the context of treatment of MPE/MPN of non-mesothelioma malignancies, including NSCLC (15-17) (Table 2).
Yokoi et al. reported the prognostic outcomes of 23 NSCLC patients who underwent EPP between 1988 and 2012, with a median survival time and 5-year survival rate of 34 months and 34%, respectively (15,16). Among 12 patients with pathologic N0-1 disease, six remained alive without disease at 4 to 288 months after surgery, for a median survival time and 5-year survival rate of 126 months and 61%, respectively, a significantly better prognosis. The authors concluded that patients with N0-1 disease and carcinomatous pleuritis might be candidates for surgical treatment, including EPP.
Yamaguchi et al. evaluated the survival of 11 NSCLC patients who underwent induction chemoradiotherapy using uracil-tegafur (UFT®, Taiho Pharmaceutical Co., Ltd., Tokyo, Japan) plus cisplatin concurrently with 40 Gy hemithorax radiation followed by EPP from 1997 to 2004 (17). The 1-, 3- and 5-year overall survival rates were 100.0%, 33.3%, and 22.2%, respectively, with a median survival time of 32.1 months. The authors commented that induction therapy using UFT plus cisplatin with hemithorax radiation, followed by EPP, was feasible for NSCLC patients with carcinomatous pleuritis. However, the impact of this treatment on overall survival remains unclear.
However, a worse outcome after surgery has also been reported. Fukuse et al. performed EPP on five patients with carcinomatous pleuritis, and four of the five patients died less than a year after surgery (5).
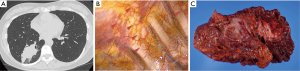
Here, we present a NSCLC patient with carcinomatous pleuritis who underwent induction systemic chemotherapy followed by EPP in our institution. A 43-year-old woman was scheduled to undergo pulmonary resection due to adenocarcinoma of the right lung. Chest computed tomography showed a 6.7 cm × 3.6 cm mass in the right lung (S9) with pleural indentation (Figure 1A). Because thoracoscopic evaluation revealed numerous MPNs in the thoracic cavity without MPE (Figure 1B), she was diagnosed with stage IV disease. Six cycles of systemic chemotherapy with cisplatin and pemetrexed was administered. After completion of chemotherapy, she underwent a right EPP with R0 resection (Figure 1C). She has been alive for 18 months without any recurrence.
Evaluation of the pleural lavage cytology
Pleural lavage cytology (PLC) is a diagnostic technique used to detect tumor cells in the pleural cavity and translate this finding to a prognostic index. PLC, which is performed at thoracotomy, is generally regarded to have a prophase of or latent carcinomatous pleuritis. In fact, many reports suggest a positive intraoperative PLC status is a prognostic factor indicating a worse outcome for patients with NSCLC (18-25) (Figure 2).
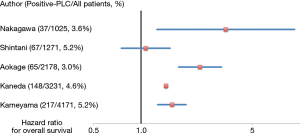
A meta-analysis performed by Saso et al. reported the association between positive PLC results and pleural, distant, and overall tumor recurrence in 4,450 patients undergoing surgical resection (20). Positive pre-resection PLC was associated with a higher rate of overall recurrence [odds ratio (OR): 4.82, 95% confidence interval (CI): 2.45 to 9.51], pleural recurrence (OR: 9.89, 95% CI: 5.95 to 16.44), distant cancer recurrence (OR: 3.18, 95% CI: 1.57 to 6.46), and unfavorable patient survival outcomes [hazard ratio (HR): 2.08, 95% CI: 1.71 to 2.52] (20). The authors suggested postoperative adjuvant chemotherapy should be considered in the management of patients with positive PLC.
Kameyama et al. reported the clinical implications of PLC using the Japanese Joint Committee of Lung Cancer Registry (19). A total of 4,171 patients were enrolled and 217 patients (5.2%) had positive PLC results. The 5-year survival was 44.5% for patients with positive PLC results and 72.8% for patients with negative PLC results, which indicated a significantly worse prognosis for patients with positive PLC results. The authors suggested an upgrade of T stage in patients with positive PLC, because the significant survival differences between patients with positive and negative PLC results disappeared when the upstage was performed.
Although negative survival influences among patients with positive PLC have been reported, pulmonary resection in these patients should not be neglected because the results of PLC do not change the T stage classification of patients according to either the 7th or the upcoming 8th TNM classification (1,26,27).
Advances in systemic chemotherapy
Several drugs can be used to treat NSCLC, including traditional and targeted chemotherapeutical agents. Targeted cancer therapies are a new class of drugs that specifically target certain molecular pathways leading to cancer phenotypes. For example, it is well known that mutations of the epidermal growth factor receptor (EGFR) gene are specifically detected in lung adenocarcinoma, with the implication that patients with this genotype are super-responders to EGFR tyrosine kinase inhibitors, such as gefitinib and erlotinib (28-30). Another treatment option is the combination of the antimetabolite pemetrexed and the angiogenesis inhibitor bevacizumab, which has been approved for the initial treatment of advanced nonsquamous NSCLCs, a specific type of NSCLC (31-34). Recently, in patients with advanced squamous and nonsquamous-cell NSCLC, significantly better survival with nivolumab, a fully human IgG4 programmed death 1 immune-checkpoint-inhibitor antibody, as compared with docetaxel, was reported (35,36). The development of the agents described above might have the potential to prolong the survival of NSCLC patients with advanced disease, including those with carcinomatous pleuritis. Consequently, the role of surgical intervention for these patients might change in the near future. However, we believe that surgical intervention has a role to play because the effect of these new drugs on survival is still unclear.
Conclusions
NSCLC patients with carcinomatous pleuritis show heterogeneous conditions. For the cases with minimal disease, which do not involve massive MPE or numerous MPNs, surgical interventions, including EPP, might have a role in improving survival. However, especially for EPP, we believe surgical intervention should be performed by experienced teams at experienced centers to minimize the morbidity and mortality associated with this radical procedure. In addition, if possible, additional prospective studies are needed to better characterize the role of surgical intervention in the multimodality treatment of patients with carcinomatous pleuritis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Webber C, Gospodarowicz M, Sobin LH, et al. Improving the TNM classification: findings from a 10-year continuous literature review. Int J Cancer 2014;135:371-8. [Crossref] [PubMed]
- Postmus PE, Brambilla E, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol 2007;2:686-93. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Ichinose Y, Tsuchiya R, Koike T, et al. The prognosis of patients with non-small cell lung cancer found to have carcinomatous pleuritis at thoracotomy. Surg Today 2000;30:1062-6. [Crossref] [PubMed]
- Fukuse T, Hirata T, Tanaka F, et al. The prognostic significance of malignant pleural effusion at the time of thoracotomy in patients with non-small cell lung cancer. Lung Cancer 2001;34:75-81. [Crossref] [PubMed]
- Ichinose Y, Tsuchiya R, Koike T, et al. Prognosis of resected non-small cell lung cancer patients with carcinomatous pleuritis of minimal disease. Lung Cancer 2001;32:55-60. [Crossref] [PubMed]
- Sawabata N, Matsumura A, Motohiro A, et al. Malignant minor pleural effusion detected on thoracotomy for patients with non-small cell lung cancer: is tumor resection beneficial for prognosis? Ann Thorac Surg 2002;73:412-5. [Crossref] [PubMed]
- Okamoto T, Iwata T, Mizobuchi T, et al. Pulmonary resection for lung cancer with malignant pleural disease first detected at thoracotomy. Eur J Cardiothorac Surg 2012;41:25-30. [PubMed]
- Fiorelli A, Santini M. In lung cancer patients where a malignant pleural effusion is found at operation could resection ever still be justified? Interact Cardiovasc Thorac Surg 2013;17:407-12. [Crossref] [PubMed]
- Kimura M, Murakami H, Naito T, et al. Outcome of platinum-based chemotherapy for non-small-cell lung cancer patients with pleural dissemination detected during surgery. Mol Clin Oncol 2013;1:949-52. [PubMed]
- Ryu JS, Ryu HJ, Lee SN, et al. Prognostic impact of minimal pleural effusion in non-small-cell lung cancer. J Clin Oncol 2014;32:960-7. [Crossref] [PubMed]
- Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 2011;6:1229-35. [Crossref] [PubMed]
- Mordant P, Arame A, Foucault C, et al. Surgery for metastatic pleural extension of non-small-cell lung cancer. Eur J Cardiothorac Surg 2011;40:1444-9. [PubMed]
- Iida T, Shiba M, Yoshino I, et al. Surgical Intervention for Non-Small-Cell Lung Cancer Patients with Pleural Carcinomatosis: Results From the Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2015;10:1076-82. [Crossref] [PubMed]
- Yokoi K, Matsuguma H, Anraku M. Extrapleural pneumonectomy for lung cancer with carcinomatous pleuritis. J Thorac Cardiovasc Surg 2002;123:184-5. [Crossref] [PubMed]
- Yokoi K, Matsuguma H. Surgical treatment of lung cancer with carcinomatous pleuritis. Nihon Geka Gakkai Zasshi 2013;114:196-200. [PubMed]
- Yamaguchi M, Ichinose Y, Shimamatsu S, et al. Preoperative concurrent chemoradiotherapy followed by extrapleural pneumonectomy for patients with non-small cell lung cancer with malignant pleural effusion and/or pleural nodules: Ten-year results of a prematurely terminated single institute phase II trial. Surg Oncol 2015;24:78-83. [Crossref] [PubMed]
- Toufektzian L, Sepsas E, Drossos V, et al. Pleural lavage cytology: where do we stand? Lung Cancer 2014;83:14-22. [Crossref] [PubMed]
- Kameyama K, Okumura N, Miyaoka E, et al. Prognostic value of intraoperative pleural lavage cytology for non-small cell lung cancer: the influence of positive pleural lavage cytology results on T classification. J Thorac Cardiovasc Surg 2014;148:2659-64. [Crossref] [PubMed]
- Saso S, Rao C, Ashrafian H, et al. Positive pre-resection pleural lavage cytology is associated with increased risk of lung cancer recurrence in patients undergoing surgical resection: a meta-analysis of 4450 patients. Thorax 2012;67:526-32. [Crossref] [PubMed]
- Aokage K, Yoshida J, Ishii G, et al. The impact on survival of positive intraoperative pleural lavage cytology in patients with non-small-cell lung cancer. J Thorac Cardiovasc Surg 2010;139:1246-52, 1252.e1.
- Shintani Y, Ohta M, Iwasaki T, et al. Intraoperative pleural lavage cytology after lung resection as an independent prognostic factor for staging lung cancer. J Thorac Cardiovasc Surg 2009;137:835-9. [Crossref] [PubMed]
- Li YN, Shi HZ, Liang QL, et al. Prognostic significance of pleural lavage cytology in patients with lung cancer: a meta-analysis. Lung Cancer 2008;60:183-92. [Crossref] [PubMed]
- Kaneda M, Yokoi K, Ito S, et al. The value of pleural lavage cytology examined during surgery for primary lung cancer. Eur J Cardiothorac Surg 2012;41:1335-41. [Crossref] [PubMed]
- Nakagawa T, Okumura N, Kokado Y, et al. Clinical relevance of intraoperative pleural lavage cytology in non-small cell lung cancer. Ann Thorac Surg 2007;83:204-8. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22.
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol 2005;23:2513-20. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34. [Crossref] [PubMed]
- Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 2013;24:20-30. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]


