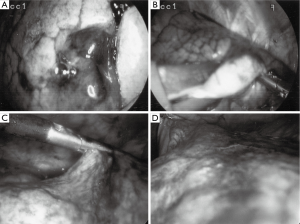
Agar blue localization of small pulmonary nodules and ground glass opacifications for thoracoscopic resection
Introduction
High resolution, low-dose radiation computed tomography (CT) used for lung cancer screening in high-risk individuals has demonstrated very encouraging early results. The Early Lung Cancer Action Project (ELCAP) results reported by Henschke et al. have sparked tremendous excitement and interest in early detection of lung cancer (1-4). One consequence of such screening has been the detection of very small pulmonary nodules and non-palpable areas visualized on thoracic CT scans, known as ground glass opacifications (GGOs) (5). A GGO is an area of focal translucency detected on the lung windows of the thoracic CT scan which may have some solid components (complex GGO) or be devoid of any solid component (pure GGO), but in either case is clearly different from solid nodules. It is currently believed that pure GGOs are markedly more indolent than complex GGOs. Size appears to have some correlation with malignant potential, as pure GGOs greater than three centimeters in size may pose a higher risk of malignancy. Volumetric growth analysis may help identify lesions worthy of biopsy; however, such capabilities are currently not widely available at most medical facilities (6). Current practice at our institution is to biopsy all lesions greater than three centimeters in size and all complex GGOs. A correlation of these lesions and bronchoalveolar carcinoma (BAC) and atypical adenomatous hyperplasia (AAH), a potential malignant precursor, has been established. Such small non-palpable areas of radiologically detected lung abnormalities pose difficulties to the thoracic surgeon attempting to biopsy and/or resect these lesions. Ideally, video assisted thoracoscopic surgical (VATS) wedge resection for frozen section analysis is performed with potential further resection (lobectomy, segmentectomy, or wide wedge resection) to follow if malignancy or AAH is identified on pathologic evaluation.
Several different approaches have been used to help localize small pulmonary nodules in preparation for resection. Non-palpable, non-visible GGOs will further exacerbate the difficulties faced in readily finding these lesions for resection. Moon, et al. has proposed injecting such lesions with contrast media under CT scan guidance then using fluoroscopy in the operating room to help localize and resect these areas (7,8). Although preliminary results are encouraging, the operative component may be cumbersome. Others have advocated the use of dye (methylene blue or indigo carmine) injected under CT scan guidance into such areas, but experience has shown that often the dye dissipates into surrounding normal lung tissue (9,10). A third alternative is the use of a Kopans hook wire for guidance, and though helpful for peripheral lesions, the efficacy drops significantly for deeper parenchymal lung lesions (11-16). Intra-operative ultrasonography may also be helpful for peripheral lung nodules, but is not very beneficial for deeper lesions or GGOs (17). Newer techniques have been proposed, such as injection of methyl macrylate in an attempt to harden the area thus rendering the lesion palpable. Concerns over the potential adverse health effects of methyl macrylate as well as the effects on local histologic architecture in these already difficult to pathologically diagnose areas have tempered widespread use of this technique.
Methods
One possible way to make resection of these areas easier is by using an inert, colorful material that could become palpable and yet not destroy the histology. In an attempt to localize several small pulmonary nodules and GGOs, liquid agar was mixed with methylene blue and was subsequently injected under CT scan guidance into the area of concern. The agar hardened, leaving a blue palpable lesion readily apparent thus easily resected. Agar has been used previously as a localizing method in human tissues and the blue dye adds visual help. The agar technique also has the added benefit of not distorting the pathologic architecture of the resected specimen. The method of powdered agar liquefaction and solidification has been well described by Tsuchida et al. and experimental animal models described (18). Purified powdered agar is dissolved in distilled water at a concentration of 5%. The agar powder is liquified using a microwave and then filtered using a 0.45 µm membrane. Five milliliters of methylene blue is added to the liquid agar and the solution is then injected percutaneously into the lung lesion using an 18 gauge needle under CT scan guidance. On deeper lesions (greater than 15 millimeters from the pleural surface) a concurrent hook wire may also be placed.
Prior to operation, all lesions were classified into three regions: peripheral (P), outer-third (O), and deep (D). Peripheral lesions were readily visible after agar blue processing (Figure 1). Outer-third and deep lesions benefited from concurrent hook wire needle localization, as an aid to guiding VATS resection of appropriate area of lung. Patients with outer-third peripheral lesions benefited from gentle (<5 mmHg pressure) insufflation of the thorax using CO2 after single lung ventilation was instituted. Care was taken to allow for an open exit port preventing any risk of induced tension pneumothorax. Areas of normal lung tissue compressed under insufflation while the lesion often dimpled out. Wedge resections were carried out, and prior to extraction from the thorax, all specimens were placed into a thoracoscopic bag extraction system to prevent tract tumor seeding. Intraoperative air leaks were checked for and if visible were controlled via standard surgical technique and application of Progel lung sealant.
Results
Forty five agar blue localizations were performed, six using concurrent hook wire needle localization. Patient demographics were as follows: twenty six males; nineteen females; ages 42 to 81 (mean age, 68); thirty eight of forty five were active tobacco smokers (average 1.5 packs per day); four had been smokers prior to quitting; and three never smoked.
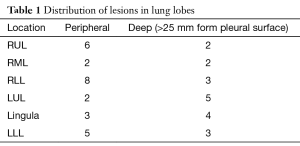
All areas localized were complex GGOs greater than two centimeters in size (four) or pulmonary nodules less than one-and-a-half centimeters in size (twelve). The distribution of lesions is shown in Table 1. All patients were placed in the lateral thoracotomy position. Twenty patients received general anesthesia and had single lung ventilation using a double-lumen endotracheal tube. Twenty five patients with peripheral lesions were operated on using spontaneous ventilation and local anesthesia using the awake video assisted thoracic surgery (AVATS) technique (19). All lesions were resected for microscopic analysis using VATS wedge resection. Thirty two patients were found to have malignancy and had completion lobectomy or segmentectomy upon frozen section demonstration of malignancy. Three patients with poor pulmonary function (FEV1 <0.7) were treated using wide wedge resection with 2 centimeter pathologically benign margins all performed thoracoscopically for microscopically demonstrated AAH. Ten patients with microscopically proven benign disease, such as granulomas, had VATS wedge resections alone. Mean chest tube duration was 8 hours. Average length of stay was 2.5 days for lobectomy/segmentectomies, and 1 day for wedge resection. All patients are doing well in follow-up. Mean follow-up was 24 months.
Full table
Discussion and conclusions
In conclusion, the careful analysis and preoperative preparation of small pulmonary nodules and GGOs using methylene blue dyed agar and selective CT scan guided needle localization significantly aides in thoracoscopic resection of these areas. The technique is simple to perform and appears to be safe. This novel technique was applied to 45 patients who had small nodules or GGOs. On the day of surgery, the lesions were visualized in entirety using agr mixed with methylene blue injected directly into the lesion under CT visualization. Once the agar set, the lesion was easily resected thoracoscopically, often using AVATS technique.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- Henschke CI. Early lung cancer action project: overall design and findings from baseline screening. Cancer 2000;89:2474-82. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF. CT screening for lung cancer. Radiol Clin North Am 2000;38:487-95. viii. [Crossref] [PubMed]
- Aberle DR, Gamsu G, Henschke CI, et al. A consensus statement of the Society of Thoracic Radiology: screening for lung cancer with helical computed tomography. J Thorac Imaging 2001;16:65-8. [Crossref] [PubMed]
- Yankelevitz DF, Henschke CI. Small solitary pulmonary nodules. Radiol Clin North Am 2000;38:471-8. [Crossref] [PubMed]
- Yankelevitz DF, Reeves AP, Kostis WJ, et al. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 2000;217:251-6. [Crossref] [PubMed]
- Moon SW, Wang YP, Jo KH, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg 1999;68:1815-20. [Crossref] [PubMed]
- Choi BG, Kim HH, Kim BS, et al. Pulmonary nodules: CT-guided contrast material localization for thoracoscopic resection. Radiology 1998;208:399-401. [Crossref] [PubMed]
- Wicky S, Mayor B, Cuttat JF, et al. CT-guided localizations of pulmonary nodules with methylene blue injections for thoracoscopic resections. Chest 1994;106:1326-8. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Waldhausen JH, Shaw DW, Hall DG, et al. Needle localization for thoracoscopic resection of small pulmonary nodules in children. J Pediatr Surg 1997;32:1624-5. [Crossref] [PubMed]
- Sartoris F, Cittadini G, Saitta S, et al. CT-guided needle localization of lung nodules for thoracoscopic resection. Eur Radiol 1996;6:420-4. [Crossref] [PubMed]
- Schwarz RE, Posner MC, Plunkett MB, et al. Needle-localized thoracoscopic resection of indeterminate pulmonary nodules: impact on management of patients with malignant disease. Ann Surg Oncol 1995;2:49-55. [Crossref] [PubMed]
- Hardaway BW, Hoffer FA, Rao BN. Needle localization of small pediatric tumors for surgical biopsy. Pediatr Radiol 2000;30:318-22. [Crossref] [PubMed]
- Zhang J, Zhao H, Fu Z. CT-guided percutaneous transthoracic fine needle aspiration biopsy of small peripheral pulmonary lesions. Zhonghua Zhong Liu Za Zhi 1997;19:127-9. [PubMed]
- Neuwirth J, Fanta J, Vojtísek O. Percutaneous localization and marking of small solitary pulmonary nodules in CT imaging before video thoracoscopy surgery. Cas Lek Cesk 1999;138:666-8. [PubMed]
- Santambrogio R, Montorsi M, Bianchi P, et al. Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann Thorac Surg 1999;68:218-22. [Crossref] [PubMed]
- Tsuchida M, Yamato Y, Aoki T, et al. CT-guided agar marking for localization of nonpalpable peripheral pulmonary lesions. Chest 1999;116:139-43. [Crossref] [PubMed]
- Klijian AS, Gibbs M, Andonian NT. AVATS: Awake Video Assisted Thoracic Surgery--extended series report. J Cardiothorac Surg 2014;9:149. [Crossref] [PubMed]