Surgery for lung cancer invading the mediastinum
Introduction
Lung cancer invading the mediastinum is currently classified as T4 disease, which without lymph nodes metastasis (N0) or limited hilar lymph node spread (N1) falls within the spectrum of stage IIIA lung cancer. Mediastinal structures invaded by lung cancer either by direct extension or lymph node metastasis include the thoracic spine, superior vena cava (SVC), aorta and supra-aortic blood vessels, the atria, esophagus and trachea. Surgery is not routinely offered to patients with such advanced presentation and is often determined on an individual basis within the confines of a multidisciplinary healthcare team. Not uncommonly, younger and stronger patients drive this decision making for such radical surgeries.
For successful outcomes, a comprehensive evaluation of the patient history, tumor histology, and if applicable, tumor response following neoadjuvant therapy, along with careful preoperative surgical planning are a must. Equally important is an honest and thoughtful conversation with the patient detailing the steps of the treatment plan. Reasonable expectations should be offered as well as appropriate alternatives to make the most suitable decision.
Below, we provide an overview of the largest series reported on lung cancer invading mediastinal structures. Management of thoracic spine invasion by lung cancer will not be discussed here, while tracheal and/or carinal involvement is mentioned elsewhere in this issue of the journal. The following represents a brief summary of the current literature as well as our experience in managing these rare and sometimes challenging surgeries.
Perioperative considerations
Extensive preoperative investigation is paramount for successful surgical outcomes of such tumors. Below is a brief synthesis of the current literature along with our experience planning for these procedures:
- Only complete resection (R0) can provide a potential opportunity for a cure;
- N2 disease is considered a very strong contraindication for resection by many leading surgeons, therefore every effort should be made to detect any N2 involvement, including PET/CT-Scans, mediastinoscopy and biopsies;
- Preoperative pulmonary function testing and adequate pulmonary reserve can help in the decision making, particularly when pneumonectomy is necessary for R0 resection;
- Imaging is essential for staging. Besides PET and CT-scans, cardiac magnetic resonance and trans-esophageal endoscopy can provide additional information regarding tumor borders and atherosclerosis in the aorta should the arch need be mobilized;
- Preoperative intravascular volume expansion and vasopressors can temporarily maintain hemodynamics during SVC clamping and reduce neurological sequelae if cardiopulmonary bypass (CPB) is not utilized. We recommend placing large bore femoral IV access preemptively should bypass need be instituted;
- A heart-lung machine, perfusionist, and possibly cardiac surgeon should be readily available if the need for CPB arises;
- Expertise in the surgical technique and postoperative care of these patients cannot be overemphasized.
Superior vena cava (SVC)
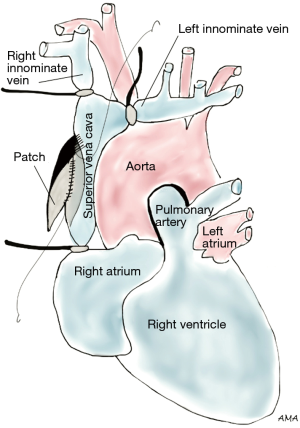
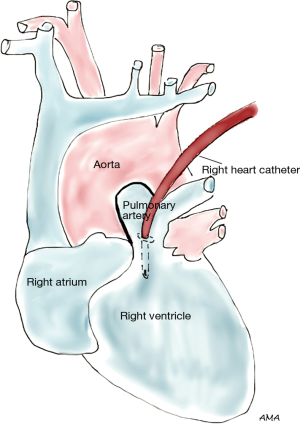
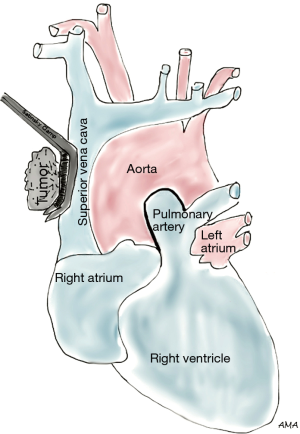
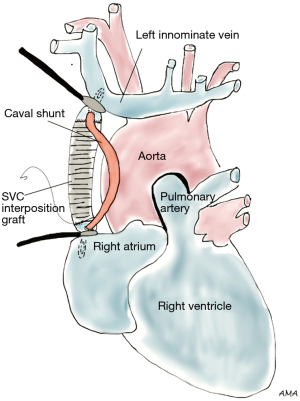
Lung cancer involving the SVC presents as a spectrum of diseases where patients may or may not develop SVC syndrome. Several approaches can be performed to gain access to the SVC. The classical approach to SVC surgery is through a median sternotomy, however, a standard right posterolateral thoracotomy can be used in most cases, particularly when SVC involvement is identified during a lobectomy. Anterior approaches such as hemiclamshell thoracotomy, cervicosternotomy or a combination of the previous incisions have been described as well for resection of central lung cancers invading the SVC. Large bore femoral intravenous access should be secured preoperatively to maintain adequate intravascular volume and hemodynamics during clamping. Lung cancers abutting the SVC can be excised by sharp dissection. If limited and/or superficial invasion of SVC is identified, the tumor can be excised via a tangential resection. A partial occlusion Satinsky clamp is used to side-bite the cava, ensuring vascular control (Figure 1). The caval wall is then removed en bloc along with a right upper lobectomy or pneumonectomy, whichever ensures complete tumor resection. The size of the SVC defect typically determines the choice for closure. Primary repair is preferred when feasible. Alternatively, a patch repair can be performed using autologous pericardium or polytetrafluoroethylene (PTFE) Gore-Tex synthetic prosthesis (Figure 2). Dacron is associated with thrombosis and should be avoided (1). Tumors invading more than 50% of the cava require complete SVC excision and replacement with a ringed PTFE graft (size 18–20 mm) or tubularized pericardium. In the event tumor extension reaches the right innominate vein, the graft can be placed between the right atrium and the left innominate vein (Figure 3). A caval shunt may be used in SVC resection and reconstruction; however, SVC can be safely clamped for 60 min without neurological sequelae based on clinical and experimental models. CPB is rarely indicated for isolated SVC invasion without other mediastinal structures. Preoperative heparinization, intravascular volume expansion and vasopressors are recommended to maintain hemodynamics during clamping (2), particularly following acute SVC obstruction when preload may be compromised. Patients with chronic SVC obstruction display extensive collateralization of flow, and typically tolerate clamping well. Long term graft patency is common after 6 months of anticoagulation (2). Many retrospective series of lung cancer invading the SVC have been published. Overall 5-year survival rates range between 11% and 31% (3-7). Morbidity can reach 40% (4,5,8) and mortality has been reported between 0% and 14% (3-6). Respiratory complications and infections are the most commonly associated complications. Similar to other T4 tumors in this review, N2 disease portends a worse prognosis (9).
Aorta
The aorta may be invaded by direct extension of left lung cancer, particularly tumors of the left upper lobe. The decision to operate on patients with potential aortic invasion relies substantially on preoperative examination, however the ability to resect is often decided after direct exploration. Efforts should be made to ensure lack of full thickness aortic wall invasion, intrapleural spreading or mediastinal lymph node metastasis, all of which are contraindications for surgical resection. Findings on preoperative imaging such as loss of fat plane between the tumor and aorta, and/or significant encroachment of the tumor on the aorta raise the suspicion for aortic invasion by cancer. If the tumor extends to the adventitia of the aorta, subadventitial dissection may be attempted. Partial resection and patch replacement can be done as well, and both procedures may not require CPB. Circumferential resection and replacement of the aorta will require total CPB, partial CPB (left heart bypass, Figure 4) or a passive bypass shunt. Arch mobilization is often required to facilitate resection. Tumors impinging on the distal arch or the proximal descending aorta may hamper arch mobilization and limit hilar dissection. The extent of pulmonary resection, lobectomy or pneumonectomy, is determined by the size and location of the primary tumor. Lung cancers extending to the aorta may be removed with acceptable clinical outcomes, yet the surgery remains associated with a very high morbidity [31% (10), 34% (11)]. Significant complications reported include postoperative bleeding, intrapleural bleeding and death (10). Five-year survival rates ranged from 31% to 48.2% (10,11). Interestingly, in the case series by Ohta et al. (10), 5-year survival rate was 70% for patients with N0 disease.
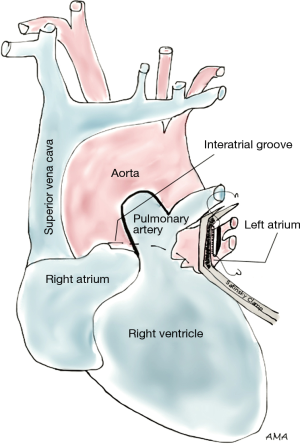
Left atrium
Lung cancers involving the left atrium typically occur as direct ingrowths into the atrial wall (with other structures often involved as well) or as a result of tumor embolization through the pulmonic veins. Most studies depict heterogeneous presentations; therefore care should be taken when interpreting their results. For R0 resection, the interatrial groove (of Sondergaard) is opened which improves access to the left atrium. Intrapericardial pneumonectomy may be required to ensure complete resection. Several authorities recommend placing patients with left atrial involvement on the heart-lung machine, defibrillating the heart, and then gaining full access to the tumor through an atriotomy. A simpler approach can be followed for limited involvement and low suspicion index for invasion beyond the wall; after dissecting the interatrial groove, a large Satinsky clamp is applied centrally on the left atrium, and the tumor can then be resected followed by primary closure with running sutures (Figure 5). This latter approach does not require CPB. Tsuchiya et al. (12) reported a series of 44 patients with left atrial invasion either to the atrium alone or in combination with other structures. Atrial clamping and primary repairs were performed in most of the cases. Ratto et al. (13) reported their experience with 19 patients who underwent atrial resection without CPB. Five-year and median survival were 14% and 25 months, respectively. There was no perioperative mortality, but morbidity was 37%. Spaggiari et al. (14) reported a 39% 3-year survival of 15 cases of limited left atrium resection, also performed without CPB. Atrial arrhythmias were the only morbidity described (15%) and 5-year survival was reported at 27% in a separate study (11). Galvaing et al. (15) reported a 44% 5-year survival in 19 patients, yet their study was associated with 11% operative mortality as well as significant morbidity, 53%.
Pulmonary artery (PA)
The main PA can be invaded directly by locally advanced lung cancer or by hilar lymph nodes abutting the PA, particularly the left main PA. Such tumors are best accessed via median sternotomy incision rather than a left thoracotomy, with CPB instituted between the venae cavae and the ascending aorta. PA resection can be performed under normothermia with a beating heart. Reconstructions using pericardium or PTFE have been reported with acceptable outcomes; however this surgery remains associated with significant morbidity. Furthermore, most reports are plagued with heterogeneity of tumor stages precluding a pure assessment of the surgical benefit. Five-year survival rates in many series have been reported between 41% and 46% (16-18). Postoperative graft thrombosis and massive hemoptysis are major complications that have been reported to occur following this surgery and can be fatal (16). Similar to all tumors in this review, N0 disease was associated with a better 5-year survival [(67% for N0 vs. 20% for N2 (16)].
Esophagus
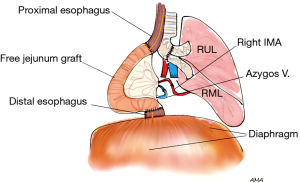
Lung cancer metastasis to the esophagus (via paraesophageal and subcarinal lymph nodes) is significantly more common than direct invasion. When involved by direct invasion with limited muscular wall involvement, enucleation can be attempted with preservation of the esophageal mucosa. However, the prognosis remains very poor due to extensive disease which often precludes R0 resection. While both pulmonary and esophageal resections are technically feasible, the compounded morbidity from such extensive resection can be challenging and is often prohibitive. Philippe Dartevelle presented at the 94th annual meeting of the American Association of Thoracic Surgery an unpublished report of a young, active 27-year-old female Olympian athlete who developed a low grade sarcoma of the right lower lobe with invasion to the bronchus intermedius and the esophagus. Access was obtained via a right thoracotomy, followed by right lower lobectomy and esophagectomy. Pulmonary reconstruction involved right upper and middle lobes bronchi side-to-side anastomosis with re-implantation into the right main bronchus, while the esophagus was replaced with a free jejunal graft anastomosed to the right internal mammary artery and azygos vein (Figure 6). Three years following the surgery, the patient remained alive with resumption of previous activities. As exemplified by this case, this surgery is reserved to a very select group of patients, where benefits may outweigh the risks.
Conclusions
In this brief review, we presented an eclectic group of tumors of the lung that invade the mediastinum. T4N0 and T4N1 lung cancers remain “surgical diseases” that warrant evaluation by general thoracic surgeons and may be amenable for a cure. Despite a lack of well-designed trials that can statistically address the benefit of surgery vs. definitive chemoradiotherapy in this population, there is sufficient anecdotal evidence to support a role for surgery in providing cure to these patients. Substantial efforts need be undertaken to rule out N2 disease prior to offering these radical surgeries. Careful and exhaustive preoperative planning as well as surgical expertise cannot be overemphasized for successful surgical outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gloviczki P, Pairolero PC, Toomey BJ, et al. Reconstruction of large veins for nonmalignant venous occlusive disease. J Vasc Surg 1992;16:750-61. [Crossref] [PubMed]
- Dartevelle PG. Herbert Sloan Lecture. Extended operations for the treatment of lung cancer. Ann Thorac Surg 1997;63:12-9. [PubMed]
- Misthos P, Papagiannakis G, Kokotsakis J, et al. Surgical management of lung cancer invading the aorta or the superior vena cava. Lung Cancer 2007;56:223-7. [Crossref] [PubMed]
- Spaggiari L, Magdeleinat P, Kondo H, et al. Results of superior vena cava resection for lung cancer. Analysis of prognostic factors. Lung Cancer 2004;44:33. [Crossref] [PubMed]
- Yildizeli B, Dartevelle PG, Fadel E, et al. Results of primary surgery with T4 non-small cell lung cancer during a 25-year period in a single center: the benefit is worth the risk. Ann Thorac Surg 2008;86:1065-75; discussion 1074-5. [Crossref] [PubMed]
- Shargall Y, de Perrot M, Keshavjee S, et al. 15 years single center experience with surgical resection of the superior vena cava for non-small cell lung cancer. Lung Cancer 2004;45:357-63. [Crossref] [PubMed]
- Lanuti M, De Delva PE, Gaissert HA, et al. Review of superior vena cava resection in the management of benign disease and pulmonary or mediastinal malignancies. Ann Thorac Surg 2009;88:392-7. [Crossref] [PubMed]
- Suzuki K, Asamura H, Watanabe S. Combined resection of superior vena cava for lung carcinoma: prognostic significance of patterns of superior vena cava invasion. Ann Thorac Surg 2004;78:1184-9; discussion 1184-9. [Crossref] [PubMed]
- Spaggiari L, Regnard JF, Magdeleinat P, et al. Extended resections for bronchogenic carcinoma invading the superior vena cava system. Ann Thorac Surg 2000;69:233-6. [Crossref] [PubMed]
- Ohta M, Hirabayasi H, Shiono H, et al. Surgical resection for lung cancer with infiltration of the thoracic aorta. J Thorac Cardiovasc Surg 2005;129:804-8. [Crossref] [PubMed]
- Spaggiari L, Tessitore A, Casiraghi M, et al. Survival after extended resection for mediastinal advanced lung cancer: lessons learned on 167 consecutive cases. Ann Thorac Surg 2013;95:1717-25. [Crossref] [PubMed]
- Tsuchiya R, Asamura H, Kondo H, et al. Extended resection of the left atrium, great vessels, or both for lung cancer. Ann Thorac Surg 1994;57:960-5. [Crossref] [PubMed]
- Ratto GB, Costa R, Vassallo G, et al. Twelve-year experience with left atrial resection in the treatment of non-small cell lung cancer. Ann Thorac Surg 2004;78:234-7. [Crossref] [PubMed]
- Spaggiari L, D' Aiuto M, Veronesi G, et al. Extended pneumonectomy with partial resection of the left atrium, without cardiopulmonary bypass, for lung cancer. Ann Thorac Surg 2005;79:234-40. [Crossref] [PubMed]
- Galvaing G, Tardy MM, Cassagnes L, et al. Left atrial resection for T4 lung cancer without cardiopulmonary bypass: technical aspects and outcomes. Ann Thorac Surg 2014;97:1708-13. [Crossref] [PubMed]
- Venuta F, Ciccone AM, Anile M, et al. Reconstruction of the pulmonary artery for lung cancer: long-term results. J Thorac Cardiovasc Surg 2009;138:1185-91. [Crossref] [PubMed]
- Mu JW, Lü F, Wang YG, et al. Surgical results of T4 lung cancer invading left atrium and great vessels. Zhonghua Yi Xue Za Zhi 2008;88:383-6. [PubMed]
- Wang XX, Liu TL, Yin XR. Surgical treatment of IIIb-T4 lung cancer invading left atrium and great vessels. Chin Med J (Engl) 2010;123:265-8. [PubMed]