Video-Assisted Thoracic Surgery Lobectomy: Results in Lung Cancer
Department of General and Thoracic Surgery, University Hospital Virgen Macarena, Seville, Spain.
|
Original Article
Video-Assisted Thoracic Surgery Lobectomy: Results in Lung Cancer
Department of General and Thoracic Surgery, University Hospital Virgen Macarena, Seville, Spain.
|
|
Abstract
Background: The application of video-assisted thoracic surgery (VATS) in major pulmonary resections is still far from routine in most hospitals,
even though the safety and technical feasibility of the procedure have by now been amply demonstrated. This paper reports on the surgical
technique used by the authors for VATS lobectomy, on their experience of the procedure and on the results obtained.
Methods: A retrospective study was performed of all patients undergoing VATS lobectomy at the our Thoracic Surgery Department ,between
1993 and 2009.The clinical records of all patients were reviewed, and the following variables were noted for purposes of analysis: patient
age and sex; clinical diagnosis; staging; date of surgery; type of surgery; conversion to conventional surgery and grounds for conversion;
duration of surgery; intraoperative, postoperative and long-term complications; postoperative stay, final diagnosis and staging; and
death rates.
Results: A total of 349 VATS lobectomies were performed over the study period (292 men, 57 women; mean age 59.7) The aetiology was
non-small-cell lung carcinoma (NSCLC) in 313 patients and benign processes in 26;four patients had carcinoid tumours, and a further six
required lobectomy due to metastases. The overall conversion rate was 9.4%. Mean duration of lobectomy was 148 minutes, and median duration
92 minutes. Mean postoperative was 3.9 days. The morbidity rate was 12.89 %, mostly involving minor complications. Perioperative
mortality was 1.43%. There were no intraoperative deaths. The overall five-year survival rate for patients with NSCLC was 80.1%.
Conclusion: VATS lobectomy is a safe and technically-viable procedure that meets oncological criteria for lung-cancer surgery. Major pulmonary
resection using VATS should be considered the procedure of choice for a number of benign processes and for early-stage bronchogenic
carcinoma (T1-T2 N0 M0).
Key words
Video-assisted thoracic surgery; VATS; lung cancer; thoracoscopy; minimally invasive surgical procedures; lobectomy
J Thorac Dis 2010;2:29-35. DOI: 10.3978/j.issn.2072-1439.2010.02.01.014
|
|
The first thoracoscopy was performed in 1910, when Hans
Christian Jacobaeus inserted a rigid cystoscope into the pleural
cavity (1); since then, the technique has undergone far-reaching
changes that could not have been foreseen by the earliest practitioners.
Major advances both in endoscopic material and in visualisation
techniques, together with the use of single-lung ventilation,
enabled Landreneau et al to lay the technical and strategic foundations
for modern video-assisted thoracic surgery (VATS) in 1992
(2).
Over the last fifteen years, major pulmonary section using
VATS has been shown to be both safe and technically-feasible, and
to offer a number of advantages over conventional surgery
(3,4,5,6,7). Nevertheless, its adoption as a standard technique is proving slow; VATS is not routinely performed in most hospitals,
and tends to be used mainly in highly-specialised centres.
Recent reviews of the outcomes obtained in large patient series
highlight the importance of training surgeons in this complex technique
(5,6). Our Department has been performing major pulmonary
resections by VATS since 1993, making it one of the pioneering
groups in Europe.
The Department also runs a twice-yearly training programme in
advanced thoracoscopic surgery at the MINIMALLY INVASIVE
SURGERY CENTRE JESUS USON in Caceres (Spain) www.
ccmijesususon.com.
Available data suggest that the proportion of lobectomies performed
using VATS in Spain is still very small, even below the European
average, although widespread interest has been expressed in
the adoption of this technique in the country's leading hospitals.
The present paper reports on the VATS lobectomy technique in
use at this institution, on the surgeons' experience with the technique
and on the outcomes obtained over a 16-year period.
|
|
Material and methods
This descriptive, retrospective review included all patients undergoing VATS lobectomy at the Thoracic Surgery Department, between 1993 and 2009. The clinical records of all patients - drawn
from the Hospital archive - were entered into the Unit database
(Microsoft R Office Access) for statistical analysis using the software
package SPSS 13.0 for Windows.
The following variables were analysed: patient age and sex;
clinical diagnosis; clinical staging; pathological staging; date of
surgery; conversion to conventional surgery and grounds for conversion;
duration of surgery; intraoperative and perioperative morbidity
and mortality; and actuarial 5-year survival rates.
Inclusion criteria
At this Department, VATS lobectomy has become the procedure
of choice for the treatment of early-stage non-small cell bronchogenic
carcinoma (NSCBC) and of a number of benign processes.
Since March 1993, several indications have been modified and
expanded, and more importantly the number of contraindications
has been reduced. The current indications for VATS lobectomy
are:
1. Tumour size < 5 cm, although, like other authors, our team
has successfully resected tumours greater than 6 cm (10,17).
2. Peripheral location, i.e. over 1 cm from the fissure and over 3
cm from the lobar carina. This is a relative criterion, since lobectomies
can safely be performed less than 1 cm from the fissure;
small series of successful sleeve VATS lobectomies have been reported
(13).
3. Stage I, N0, although is this is not a totally exclusive criterion;
metastatics intrapulmonary or mediastinal lymph nodes detected
intraoperatively do not necessarily contraindicate resection, although
they may difficult it.
4. Open fissures, although for certain lobectomies (e.g. right upper),
this is not absolutely essential.
Exclusion criteria or contraindications for VATS lobectomy
have varied over the years, becoming less restrictive as the Unit's
experience has grown. They currently include chest wall invasion,
tumour infiltration beyond the fissure, invasion of the pericardium
or diaphragm, and neoadjvant radiotherapy or chemotherapy; the
latter is also relative, since VATS lobectomy has been shown to be
safe and technically viable in patients receiving induction
chemotherapy (21,22), although it might hinder dissection.
Surgical technique:
The patient is placed in the lateral decubitus position, and selective
intubation is used in all cases. Three entry-port incisions are
made to introduce 12 mm trocars: one in the seventh or eighth intercostal
space (depending on patient thorax configuration) in the
midaxillary line, for the camera; a second just below the scapular
vertex in the sixth or seventh intercostal space; and the third in the
third or fourth intercostal space in the anterior axillary line. Having
confirmed the viability of the technique, an anterior minithoracotomy approximately 4-5 cm long is placed over the fifth intercostal
space, without rib spreading, for the insertion of surgical instruments
and dissection of vessels and bronchi. The surgeon stands
facing the patient, with the principal nurse beside him.Two assistants
are placed behind the patient; the more caudally-located of
the both operates the camera (Fig.1).
Exploratory videothoracoscopy is performed to rule out any unforeseen
causes of inoperability (e.g. pleural carcinomatosis with
no pleural effusion) (10,18,34), and to check tumor size and the absence
of fissure invasion. The presence of pleural adhesions or
small hilar lymph-node swellings is not considered a contraindication
for VATS resection.
Anatomical pulmonary resection using VATS entails the individual
dissection, stapling and sectioning of the pulmonary vein,
pulmonary arteries and bronchi. The superior pulmonary veins are
dissected and sectioned prior to artery dissection; this is not essential
in the case of the inferior pulmonary veins. Systematic dissection
of mediastinal lymph nodes is routinely performed, as in conventional
open surgery; the number of lymph nodes removed is
similar in our experience to that of conventional surgery (Fig. 2,3,4).
Figure 2 Dissection and stapling of upper lobe pulmonary vein and anterior
arterial trunk in the right
upper lobectomy
|
|
Results
A total of 349 VATS lobectomies were performed between
March 1993 and December 2009. The ratio of male to female patients
was 6:1 (292:57). Mean age was 59.7 (range 12-84, mean 63;
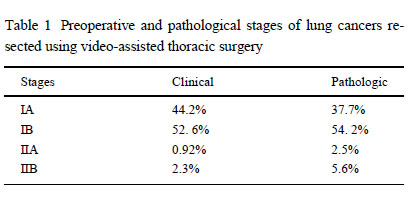
median 61). A total of 313 cases were diagnosed as non-small cell
bron chogenic carcinoma (NSCBC); clinical and pathological
stages are shown in Table 1. Twenty-six patients had benign processes
(e.g. pulmonary sequestration, cystic adenomatoid malformation,
bronchiectasis,), 4 patients had carcinoid tumors and 6 pa
tients required lobectomy due to metastases that ruled out wedge
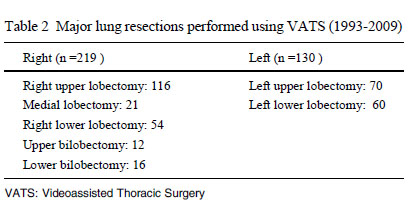
resection. The number of patients undergoing each type of lobectomy
is shown in Table 2; right upper lobectomy was by far the most
common type.
During this period, 22 VATS pneumonectomies were also performed;
these are not included, in order to ensure a more uniform
sample. There are very few indications for VATS pneumonectomy,
and the operation itself more than the VATS technique would
increase morbidity/mortality, as in open surgery.
Conversion to conventional surgery was more frequent in the early,
learning years. The overall conversion rate was 9.4% (n = 33), due
to heavy bleeding in 13 cases always stopped prior to conversion
and to technical difficulties (e.g. extensive and/or very firm adhesions,
calcified lymph nodes) in 19 cases. One further patient was
converted due to invasion of the pulmonary artery (confirmed after
vein sectioning). Mean duration of lobectomy was 148 minutes,
but the median duration was 92 minutes, since the procedure was
slower at first than in later years.
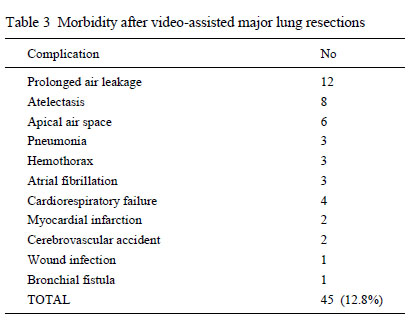
Mean postoperative stay was 3.9 days. The morbidity rate was
12.8 %, mostly involving minor complications (the most common
being air leak lasting longer than four days), as shown in Table 3.
Perioperative mortality (i.e. up to 30 days post-surgery) was 1.43%
(2 cases of sepsis and 1 case each of acute myocardial infarction,
pneumonia and cardiorespiratory failure). There were no intraoperative
deaths.
The acturial 5-year survival rate for patients with NSCBC was
80.1%. During follow-up, three patients displayed mediastinal recurrence
(despite the removal of mediastinal lymph glands from all
patients), one developed cerebral and costal metastases three
months post-surgery, two developed metachronic tumours 23 and
48 months postsurgery, respectively; three patients displayed same-lung recurrence. There were seven cases of brain metastasis
and seven of multiple metastases.
   |
|
Discussion
The American College of Chest Physicians, in its evidence-
based clinical practice guidelines, suggests that "In patients
with stage I NSCLC who are considered appropriate candidates for
thoracoscopic anatomic lung resection (lobectomy or segmentectomy),
the use of video-assisted thoracic surgery by surgeons experienced
in these techniques is an acceptable alternative to open thoracotomy"
(13). Yet at present few centres consider this to be the
procedure of choice for the treatment of early-stage lung cancer.
Over the last few years, a large number of case reviews have
been published strongly suggesting that patients undergoing VATS
lobectomy experience less postoperative pain, reduced chest tube
time, fewer perioperative complications and shorter hospital stays
than those undergoing conventional surgery
(3,4,6,7,10,17,18,19,30,32,44,46,48,50,51,52,53). Other reported
advantages over open surgery include improved quality of life, particularly
in the first year (46), and reduced immunological aggression,
evident in lower IL-6 and PCR levels compared to open
surgery (15,18,44). Other authors also report fewer delayed or reduced
doses of chemotherapy in patients receiving adjuvant
chemotherapy (21).
VATS lobectomy should therefore be the therapeutic approach
of choice in patients with early-stage NSCLC.
In this series, there were no intraoperative deaths, and perioperative
mortality in the 349 patients undergoing major pulmonary resection
using VATS was very low (1.43%), a finding also reported
for other large series (3,4,5,7,17).
Although the learning curve has to an extent been rightly blamed for increased morbidity and mortality in this type of
surgery (7), this should no longer happen, since the technique is
becoming increasingly standardized and detailed, and can be taught
and practiced at specialist training centers until thorough mastery is
achieved. There is no single standard technique for VATS lobectomy, but variations between schools tend to be very minor. The
greatest differences relate to the number and placement of trocars
and the minithoracotomy, the position of the surgeon behind or
facing the patient, and the use of a 0? versus 30? optic (49).
Most surgeons perform an anatomical dissection of vascular and
bronchial structures, respecting the surgical and oncological principles
of conventional thoracotomy.
Although Lewis and Caccavale reported excellent results in
1998 using simlutaneous stapling with no individual dissection, the
present authors feel that this technique should only be used when
technically required (32). At this department, the technique has only
been used once, to perform a left lower lobectomy in a high-risk
tetraplegic patient with a T1N0M0 bronchogenic carcinoma, displaying
a totally open fissure, but with the vein dissected isolated.
The rate of conversion to conventional surgery was 9.4%; the rate
was naturally higher in the early, learning years. Similar results are reported by other authors, including Shaw et al (47). The mean duration
of surgery is another variable which has decreased as surgeons
become more familiar with VATS resection; median surgery
time is currently 92 minutes, a figure rather lower than that noted
by other authors (30,40).
Survival rates for VATS patients appear to be similar to, or better
than, those reported for conventional surgery. Sugi et al reported
a 5-year survival rate of 90% for VATS lobectomy, compared
with 85% for conventional lobectomy (39). Nevertheless, these results
must be viewed with a degree of caution, due to a possible
implicit bias at patient selection. The 5-year survival rate for the
present series was 80.1% for patients with N NSCLC; this figure is
slightly higher than the 77% reported by Mckenna et al and the
78% observed by Onaitis et al (7,45).
Despite the lack of evidence based on large-scale randomized
studies comparing videothoracoscopic procedures with conventional
surgery the authors firmly believe that if VATS surgery is properly
performed, with resection of mediastinal lymph nodes
(50,51,52,41), and meets oncological criteria for lung cancer
surgery, long-term survival should not be affected by the choice of
surgical approach. Watanabe et al reported that the number of
lymph nodes removed following VATS lobectomy was similar to
that of conventional surgery (36).
To conclude, VATS lobectomy should be the treatment of
choice for early-stage NSCLC (T1-T2N0-M0), and also for certain
benign pathologies, although further prospective, randomized studies
are required to confirm the evidence provided by the large patient
series published to date.
|
|
References
Cite this article as: Loscertales J, Valenzuela FQ, Congregado M, Merchán RJ, Varela GG, Ramírez AT, Moreno-Merino SB, Cózar Bernal F. Video-Assisted Thoracic Surgery Lobectomy: Results in Lung Cancer. J Thorac Dis 2010;2:29-35. doi: 10.3978/j.issn.2072-1439.2010.02.01.014
|