
Lymphangioleiomyomatosis: current and future
Introduction
Lymphangioleiomyomatosis (LAM) is a rare, progressive, systemic disease, which mainly affects young women. It is characterized by the proliferation of abnormal, smooth muscle-like LAM cells in the lung, inducing the destruction and cystic remodeling of the pulmonary tissue (1,2). The extra pulmonary manifestations of LAM include thoracic and abdomen lymphadenopathy caused by the infiltration of LAM cells into the lymph vessels, which induces obstruction, development of fluid filled cysts in the chest, abdomen and pelvis, lymphangioleiomyomas, and in advanced disease chylous ascites and chylothorax. The kidneys are also often involved, with angiomyolipomas (AML’s), found to a lesser degree in the spleen and liver (3,4).
LAM may occur sporadically (S-LAM), but at about 40% occurs in women with Tuberous Sclerosis Complex (TSC-LAM), an autosomal dominant multi organ genetic disorder, presenting with neurological symptoms such as autism and mental retardation, seizures, and involvement of skin, heart (cardiac rabdomyomas) and the kidneys (AML’s) (5,6). This syndrome derives from the mutation in the TSC-1 suppressor gene located in the 9q34 chromosome and which encodes hamartin protein. On the other hand, LAM presents mutation in the TSC-2 gene located in the 16p13 chromosome, encoding tuberin protein. Hemerin and tuberin are responsible for cell proliferation and growth, through the suppressor of mammalian target of rapamycin (mTOR), a serine-threonine kinase that regulates cell cycle and size. The deficit of these proteins leads to the activation of mTOR that increases cell proliferation and size (6,7).
LAM affects young women of childbearing age, with an average onset age of 34 years. Cases have also been described of women affected post menopausally, who however were under estrogen therapy (8).
The disease usually manifests with pulmonary symptoms, progressive dyspnea on exertion, pneumothorax and chylous pleural effusions. Occasionally, symptoms such as haemoptysis, cough and chyloptysis, may be present (3,8). The extra- pulmonary manifestations may involve the kidneys with presence of angiomyolipomas, which can cause abdominal hemorrhage, ascites, and abdominal tumors, lymphangioleiomyomas that can simulate ovarian cancer, abdominal sarcomas and lymphomas (4,7).
The X-Ray at the early disease usually appears normal or will show the presence of pneumothorax. For the diagnosis it is necessary to perform a high resolution CT scan (HRCT) that demonstrates the presence of multiple well-defined, thin-walled cysts with diameter from 0.2 to 2 cm in both lungs (1,8). On the other hand abdominal CT is necessary to confirm the presence of AML’s and lymphangiomyomas. In most cases pulmonary tissue biopsy is required and is the gold standard for the diagnosis especially if the HRCT does not have the typical findings of LAM (8,9). The biopsy is usually obtained by video-assisted thoracoscopy. The specimen presents nodular LAM cell focuses that infiltrate lymphatics and new cysts. These focuses may contain two types of LAM cells, epithelioid and fusiform, that presents immunohistochemical staining for the alpha-actin, vimentin, desmin and the human melanoma black 45 (HMB 45), a monoclonal antibody that reacts with enzymes involved in melanogenesis (1,8).
Most recent studies focus on biomarkers expressed by LAM cells that may be useful inducing a non-invasive diagnostic method. Podoplanin and its ligand D2-40 is expressed by pulmonary nodules and LAM cells. Vascular endothelial growth factor C (VEGF-C) and VEGF-D and the receptor VEGFR3 situated in lymphatic vessels are responsible for lymphangiogenesis, presented in patients with LAM (10,11). It was observed that VEGF-D was elevated in patients with LAM (>800 pg/mL) compared to healthy individuals, and this data combined with a typical HRCT images for LAM may help in the future for a non-invasive diagnosis (9).
LAM is a disease that affects young women, and is aggravated when taking contraceptives and during pregnancy. This fact led to anti estrogenic treatments such as tamoxifen, gonadotropine-hormone analogues, progesterone and also oophorectomy. Unfortunately the benefit of this treatment is not clear yet (12-15). The most recent trials focus on treatments with mTOR inhibitor, rapamycin, doxycycline and aromatase inhibitors. However lung transplantation remains the best treatment in the advanced phase of the disease. Supportive care with oxygen and bronchodilatators is very important, as well as the treatment of extra pulmonary manifestations, AML’s, chylothorax and ascites (3,12).
LAM molecular pathways
Most recent studies in LAM focus on the understanding of LAM pathogenesis, molecular pathways and new treatments that may affect them (16).
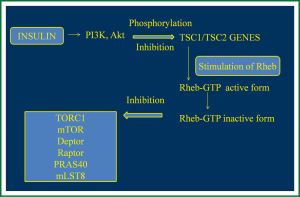
LAM occurs sporadically with prevalence of about 1 in 400,000 females, or in women with Tuberous Sclerosis Complex (TSC) at about 40%. TSC is an autosomal dominant genetic disease with a prevalence of about 1 in 6,000 children (6,17) consisting of neurological disorders and characterized by mutation in TSC1 gene encoding hamartin, a protein that participates in the reorganization of actin cytoskeleton. LAM is caused by mutation in TSC2 gene encoding tuberin that participates in cell proliferation by inhibiting the cell cycle. Hamartin and tuberin cytosolic complex participate in cell growth and in cell proliferation, through insulin or mitogen-activated protein kinase (MAPK) pathways that normally inactivate mTOR complex (18). In particular, in normal cells, growth factors like insulin activate phosphor-inositide 3-kinase (PI3K), and the protein kinase B (Akt) which phosphorylates and so inhibits the activity of TSC1/TSC2 complex (4). This complex stimulates the Ras homolog enriched in brain (Rheb) conversion from Rheb-GTP (active form) to Rheb-GDP (inactive form) and so inhibits mTOR complex 1 (TORC1) including mTOR, Deptor, Raptor, PRAS40 and mLST8 (5,7). TORC1 is responsible for cell proliferation, cell nutrition inducing protein synthesis and so cell growth, via phosphorylation of S6 kinase 1 (S6K1) and 4E-BP1. A mutation or deficit in TSC1 or TSC2, like LAM, induces defects in hamartin and tuberin function and as a result Rheb and so mTOR remains activated leading to cell proliferation and growth (1,4,7,8) (Figure 1).
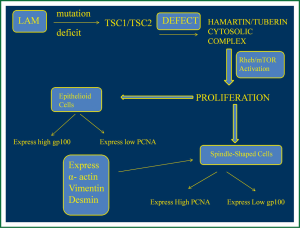
LAM is characterized by lung lesions consisting of LAM cells organized in nodules along the pulmonary lymphatics and blood vessels and near pulmonary cysts. These focuses consist of two types of LAM cells, spindle-shaped cells with higher proliferative capacity that express α-actin, vimentin and desmin, which are smooth muscle specific proteins, and may often express positivity in proliferating cell nuclear antigen (PCNA), a marker that shows mitotic activity and cell proliferation (19,20). Epithelioid cells are larger, polygonal and seem to react with human melanoma black 45 (HMB45), a monoclonal antibody which binds to gp100, a glycoprotein expressed by melanoma cells and immature melanocytes (21). The expression of gp100 and PCNA by LAM cells is not clear yet, however studies have shown that epithelioid subtype located in the periphery of the lung nodules express high gp100 and lower PCNA, while spindle like cells located in the centre of the nodules present high PCNA expression and low gp100, meaning that may represent the proliferative potential of the nodules (19,21) (Figure 2).
Pulmonary and radiologic manifestations
The most common symptoms in patients with LAM are pulmonary symptoms, due to replacement of normal pulmonary tissue by thin-walled cysts. A possible mechanism of lung degradation and cyst formation is that LAM cells, usually the spindle-shaped ones, express metalloproteinases (MMP’s) such as membrane type 1 (MT1 MMP) and matrix metalloproteinase 2 (MMP2) and catepsine-K which degrade the extracellular matrix protein and as a result facilitate cell migration. LAM cells also secrete VEGF-C, which promotes lymphangiogenesis and induces further cell migration and invasion of the lung parenchyma. The proliferation of LAM cells in the lymphatics may cause airway obstruction and air trapping that can induce cyst formation (21,22).
Dyspnea is the result of airway obstruction and cystic degradation of the parenchyma, and is the most common symptom of the disease with slow and progressive manifestation (19,23). The presence of pneumothorax is also common at the time of diagnosis, and a large proportion of patients may have had pneumothorax in their medical history. Other common symptoms are cough, chylothorax, chyloptysis and haemoptysis. The most important mechanism for the establishment of chylothorax is the obstruction of the lymphatics, while the obstruction of blood vessels leads to the formation of focal areas of hemorrhage and haemoptysis (21). The extra-pulmonary manifestations of the disease may be renal angiomyolipomas, slowly growing masses from 1 mm to 20 cm that can be asymptomatic or can lead to renal failure, hematuria, flank pain and retroperitoneal hemorrhage (24). Lymphangioleiomyomas can occur in the abdomen, pelvis and retroperitoneum with correlated symptoms such as abdominal distension and nausea. In patients that have TSC, neurological symptoms are also common.
In the early stages of the disease diagnosis may be difficult. Chest X-ray is usually normal, or presents pleural effusion and pneumothorax. The pulmonary function test can be normal but in the majority of patients shows pulmonary obstruction, although total pulmonary capacity is preserved (8). The diagnosis of LAM requires an HRCT scan that demonstrates diffuse round or ovoid thin-walled cysts, with size from a few millimeters to 3 cm, surrounded by normal pulmonary parenchyma and in advanced stages of the disease can cause total replacement of the lung tissue. Focal areas of ground glass opacities due to hemorrhage, LAM cell proliferation or hemosiderosis may also be present. The coexistence of lymphatic obstruction can induce septal thickening (17,25). An abdomen CT is also necessary to examine the presence of AML’s, lymphangiomas and chylous ascites. A tissue biopsy is usually necessary to confirm the diagnosis especially in LAM in advanced stages. However, the recent European Respiratory Society (ERS) criteria indicate that per confirm LAM is not necessary lung biopsy, when HRCT has the typical LAM image and there is evidence of one of the following: angiomyolipomas, chylous effusion, probable or definite TSC or lymphangioleiomyomas (3).
Differential diagnosis with other cystic lung diseases must be done. The most common is emphysema, where LAM cells are absent, the lungs are enlarged and the cystic airspaces are not uniform or well defined. Langerhans cell histiocytosis presents early nodules in the right mid lode and upper lobes, irregular thick-walled cysts and infiltration of Langherhans cells and eosinophils. Follicular bronchiolitis and lymphocytic interstitial pneumonia (LIP) usually presents perivascular cysts, usually lesser than LAM, and is associated with Sjögren syndrome, LES, and cardiovascular disease. Birt-Hogge-Dubbè syndrome is a rare disease, which presents skin lesions and subpleural lentiform cysts (1,17,25). LAM can also mimic lesions like light chain deposition disease (LCDD), hyper-IgE syndrome and tumors of smooth-muscle tumors such as endometrial stromal sarcoma, leiomyosarcoma and ovarian tumors, which present pulmonary metastatic cysts (1,19).
Current and future perspectives of LAM treatment
At present, there is no effective treatment for LAM (19). The disease affects women of reproductive age and worsens during pregnancy or therapy with exogenous oestrogens and contraceptive pills (26). LAM cells may also express estrogen-α and progesterone receptors (ER and PR), that activate MAPK pathways that stimulate cell growth (27,28). These facts have led to several anti-estrogenic therapies such as the use of progesterone, gonadotrhopin-releasing-hormone (GnRH) analogues such as triptorelin and goserelin and oophorectomy, but only the use of progesterone and oophorectomy seems to stabilize and improve the disease in a small group of patients, based on a number of case reports and clinical studies (19,29-31). There are no randomized placebo controlled trials that confirm the efficacy of progesterone and hormonal treatment, and the ERS criteria does not recommend its use as a routine treatment (3,12,19).
The understanding of the molecular pathways involved in LAM pathogenesis has led to new drugs for the treatment of LAM such as sirolimus (rapamycin), doxycycline. Sirolimus is an immunosuppressant approved by the FDA that inactivates mTOR complex by imitating tuberin and as a result inhibits cell proliferation (32). Phase I and II trials have also demonstrated that treatment with sirolimus was associated with a reduction of angiomyolipoma volume in patients with TSC and sporadic LAM treated with sirolimus for 12 months (33-35). The MILES study has demonstrated that therapy with rapamycin for one year induced stabilization of FEV1 and FVC, improvement of symptoms and of the quality of life. However, after discontinuation of the treatment, the benefits of rapamycin were reversed after 24 months (36). The most common adverse events during rapamycin therapy were nausea, diarrhea, mucositis, respiratory infections and hypercholesterolemia, resolved with local treatment or reduction of the sirolimus dose (36,37). MILES study have also demonstrated a reduction of serum levels of vascular endothelial growth factor D (VEGF-D) which is implicated in lymhangiogenesis in LAM, in response to the treatment and that this reduction remains even after drug withdrawal (11,36,38). Recent studies have shown a reduction of chylous effusions, lymphangioleiomyomas and improvement of the lung function during sirolimus treatment (39), however it needs further studies to determine the optimal duration of the therapy with rapamycin, the long term risks and benefits of sirolimus and confirm if VEGF-D levels can help to evaluate the disease severity and the response of the treatment with sirolimus (33,36,39).
Doxycycline is a tetracycline that inhibits MMP’s, particularly MMP-2 and MMP-9, and so remodels the extracellular matrix, induces alteration in enzymatic activity and modulates cell proliferation (40). Studies have shown an improvement of lung function and oxygen’s levels during exercise, as well as a reduction of MMP’s level in the urine, after treatment with doxycycline (41,42). Although doxycycline is a well tolerated medication, but more studies must be done to confirm its efficacy in LAM treatment.
Recently aromatase inhibitors such as letrozole have been proposed as a possible therapy approach. The inhibition of aromatase, an enzyme that converts androgens in estrogens, prevents the synthesis of estrogen and so may have a benefit in patients with LAM (12).
For advanced stages of the disease and severe airway obstruction lung transplantation remains the best treatment option with one year survival at about 79% and most common complications the presence of hemorrhage, pleural adhesions and complications related to previous surgeries (43,44).
The continuing understanding of the cellular, molecular and genetic pathways implicated in LAM is necessary for the development of new treatments. The use and discovery of new biomarkers are also essential for the prognosis and evaluation of disease progression and treatment response (1,19,44). More clinical trials must be performed to investigate new drugs, benefits, risks and early prevention of the disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Meraj R, Wikenheiser-Brokamp KA, Young LR, et al. Lymphangioleiomyomatosis: new concepts in pathogenesis, diagnosis, and treatment. Semin Respir Crit Care Med 2012;33:486-97. [PubMed]
- Ando K, Tobino K, Kurihara M, et al. Quantitative CT analysis of small pulmonary vessels in lymphangioleiomyomatosis. Eur J Radiol 2012;81:3925-30. [PubMed]
- Johnson SR, Cordier JF, Lazor R, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010;35:14-26. [PubMed]
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008;133:507-16. [PubMed]
- Yu J, Parkhitko AA, Henske EP. Mammalian target of rapamycin signaling and autophagy: roles in lymphangioleiomyomatosis therapy. Proc Am Thorac Soc 2010;7:48-53. [PubMed]
- Liu F, Lunsford EP, Tong J, et al. Real-time monitoring of tumorigenesis, dissemination, & drug response in a preclinical model of lymphangioleiomyomatosis/tuberous sclerosis complex. PLoS One 2012;7:e38589. [PubMed]
- Taveira-DaSilva AM, Moss J. Progress in the treatment of lymphangioleiomyomatosis: from bench to bedside. Rev Port Pneumol 2012;18:142-4. [PubMed]
- Johnson SR. Lymphangioleiomyomatosis. Eur Respir J 2006;27:1056-65. [PubMed]
- Chang WY, Cane JL, Blakey JD, et al. Clinical utility of diagnostic guidelines and putative biomarkers in lymphangioleiomyomatosis. Respir Res 2012;13:34. [PubMed]
- Glasgow CG, El-Chemaly S, Moss J. Lymphatics in lymphangioleiomyomatosis and idiopathic pulmonary fibrosis. Eur Respir Rev 2012;21:196-206. [PubMed]
- Xu KF, Zhang P, Tian X, et al. The role of vascular endothelial growth factor-D in diagnosis of lymphangioleiomyomatosis (LAM). Respir Med 2013;107:263-8. [PubMed]
- Casanova A, Ancochea J. Lymphangioleiomyomatosis: new therapeutic approaches. Arch Bronconeumol 2011;47:579-80. [PubMed]
- Schiavina M, Di Scioscio V, Contini P, et al. Pulmonary lymphangioleiomyomatosis in a karyotypically normal man without tuberous sclerosis complex. Am J Respir Crit Care Med 2007;176:96-8. [PubMed]
- Kim NR, Chung MP, Park CK, et al. Pulmonary lymphangioleiomyomatosis and multiple hepatic angiomyolipomas in a man. Pathol Int 2003;53:231-5. [PubMed]
- Aubry MC, Myers JL, Ryu JH, et al. Pulmonary lymphangioleiomyomatosis in a man. Am J Respir Crit Care Med 2000;162:749-52. [PubMed]
- Cottin V, Archer F, Leroux C, et al. Milestones in lymphangioleiomyomatosis research. Eur Respir Rev 2011;20:3-6. [PubMed]
- Zhang X, Travis WD. Pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med 2010;134:1823-8. [PubMed]
- Taveira-Dasilva AM, Steagall WK, Moss J. Therapeutic options for lymphangioleiomyomatosis (LAM): where we are and where we are going. F1000 Med Rep 2009;1:93.
- Harari S, Torre O, Moss J. Lymphangioleiomyomatosis: what do we know and what are we looking for? Eur Respir Rev 2011;20:34-44. [PubMed]
- Matsumoto Y, Horiba K, Usuki J, et al. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol 1999;21:327-36. [PubMed]
- Juvet SC, McCormack FX, Kwiatkowski DJ, et al. Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. Am J Respir Cell Mol Biol 2007;36:398-408. [PubMed]
- Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol 2004;28:1007-16. [PubMed]
- Ansótegui Barrera E, Mancheño Franch N, Vera-Sempere F, et al. Lymphangioleiomyomatosis. Arch Bronconeumol 2011;47:85-93. [PubMed]
- Bissler JJ, Kingswood JC. Renal angiomyolipomata. Kidney Int 2004;66:924-34. [PubMed]
- Seaman DM, Meyer CA, Gilman MD, et al. Diffuse cystic lung disease at high-resolution CT. AJR Am J Roentgenol 2011;196:1305-11. [PubMed]
- Yano S. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous oestrogen used for infertility treatment. Thorax 2002;57:1085-6. [PubMed]
- Yu JJ, Robb VA, Morrison TA, et al. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci U S A 2009;106:2635-40. [PubMed]
- Yu J, Astrinidis A, Howard S, et al. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol 2004;286:L694-700. [PubMed]
- Hohman DW, Noghrehkar D, Ratnayake S. Lymphangioleiomyomatosis: A review. Eur J Intern Med 2008;19:319-24. [PubMed]
- Contini P, Schiavina M, Schiavina R, et al. Efficacy of hormonal suppression in a patient with chyluria due to lymphangioleiomyomatosis. Multidiscip Respir Med 2011;6:313-7. [PubMed]
- Taveira-DaSilva AM, Stylianou MP, Hedin CJ, et al. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest 2004;126:1867-74. [PubMed]
- Casanova A, María Girón R, Acosta O, et al. Lymphangioleiomyomatosis treatment with sirolimus. Arch Bronconeumol 2011;47:470-2. [PubMed]
- Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 2008;358:140-51. [PubMed]
- Davies DM, de Vries PJ, Johnson SR, et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res 2011;17:4071-81. [PubMed]
- Cabrera López C, Martí T, Catalá V, et al. Effects of rapamycin on angiomyolipomas in patients with tuberous sclerosis. Nefrologia 2011;31:292-8. [PubMed]
- McCormack FX, Inoue Y, Moss J, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 2011;364:1595-606. [PubMed]
- Neurohr C, Hoffmann AL, Huppmann P, et al. Is sirolimus a therapeutic option for patients with progressive pulmonary lymphangioleiomyomatosis? Respir Res 2011;12:66. [PubMed]
- Dabora SL, Franz DN, Ashwal S, et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF- D levels decrease. PLoS One 2011;6:e23379. [PubMed]
- Taveira-DaSilva AM, Hathaway O, Stylianou M, et al. Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus. Ann Intern Med 2011;154:797-805, W-292-3.
- Chang WY, Clements D, Johnson SR. Effect of doxycycline on proliferation, MMP production, and adhesion in LAM-related cells. Am J Physiol Lung Cell Mol Physiol 2010;299:L393-400. [PubMed]
- Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med 2006;354:2621-2. [PubMed]
- Pimenta SP, Baldi BG, Acencio MM, et al. Doxycycline use in patients with lymphangioleiomyomatosis: safety and efficacy in metalloproteinase blockade. J Bras Pneumol 2011;37:424-30. [PubMed]
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European experience. J Heart Lung Transplant 2009;28:1-7. [PubMed]
- Taillé C, Borie R, Crestani B. Current management of lymphangioleiomyomatosis. Curr Opin Pulm Med 2011;17:374-8. [PubMed]


