Pulmonary hypertension: evolution of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension
On May 16th, 2016, Nick H. Kim, MD [University of California San Diego (UCSD), Division of Pulmonary, Critical Care & Sleep Medicine] delivered his keynote presentation outlining the current state of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). As the title underscores, the lecture is not simply an outline of current therapeutic strategies but is instead a blueprint detailing the historical foundation of the field, ongoing changes in clinical practice and future directions for advancement.
Dr. Kim’s talk begins with a history of the field of pulmonary vascular medicine. This outline spans from the theory of the porous heart described by Galen in the 2nd century, to the more accurate proposal of the pulmonary circulation by Ibn al-Nafis in the 13th century, to the confirmation of the pulmonary capillary network leading to the modern description of the cardiovascular system by Servetus and Harvey 300 years later (1). The earliest pathological description of pulmonary hypertension can be traced back to the German physician Ernst Von Romberg who described “pulmonary vascular sclerosis” in 1891. Several decades later his fellow countryman, Werner Forssman, introduced a new method for the diagnosis of pulmonary hypertension when he performed the first right heart catheterization on himself. This new diagnostic method opened the way for pioneers Andre Cournard and Dickinson Richards to expand our understanding of the pulmonary circulation and pulmonary vascular disease (2). The contributions by Forssman, Cournard and Richards eventually won them the Nobel Prize in Medicine. In the 1950’s, David Dresdale, a trainee of Cournand and Richards, coined the term “primary pulmonary hypertension”—the disease we now refer to as idiopathic PAH.
Through this historical overview Dr. Kim argues that pulmonary hypertension is a relatively young disease which received little attention in the first few decades following its recognition. It was not until the epidemic of primary pulmonary hypertension caused by the appetite suppressant aminorex in 1965 that the condition first received public attention. In 1973, one year after aminorex was removed from the market, the World Health Organization (WHO) formed the 1st symposium on primary pulmonary hypertension in Geneva, Switzerland. In 1982, the first treatment for primary pulmonary hypertension was published in the form of heart-lung transplantation by Norm Shumway and colleagues (3). The first medical therapy was not realized until the Nobel Prize winning work on prostacyclin by Vane, Bergstrom and Samuelson. Even after the beneficial effects of intravenous prostacyclins were first demonstrated in a later publication in 1982, thirteen years passed before epoprostenol was finally approved by the FDA for the treatment of primary pulmonary hypertension (4). Subsequent discoveries in endothelin biology and the nitric oxide pathway enabled the development of additional therapeutic agents (5).
The growth in the number of potential therapeutic targets ushered in multiple clinical trials in pulmonary hypertension. Over the past two decades many new therapies for PAH were studied and later approved starting with intravenous epoprostenol in 1995 (Figure 1) (6). During this same period the research community transitioned from undertaking mainly small, short term, surrogate-endpoint driven trials including <300 individuals to significantly larger, longer term and event-endpoint driven trials such as Seraphin (N=742), Ambition (N=500), and Griphon (N=1,156). Short-term trials designed to reach a surrogate endpoint (such as improvement in six minute walk distance) are economical and more feasible. Alternatively, long-term, event-endpoint driven trials can offer more robust argument based on morbidity and mortality data (Table 1) (6). These trials, however, require substantially more resources and may make it too difficult for new therapies to be approved for this relatively rare disease.

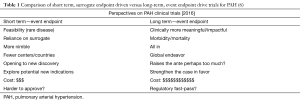
Full table
With regards to the future of PAH Dr. Kim posits that we can continue to help our patients by thinking broadly in the clinic and in the laboratory. He emphasizes that patients commonly do not receive timely or accurate diagnoses and are often not treated by pulmonary hypertension specialists leading to inappropriate or delayed therapy (7). The optimal strategy and timing for initiating medication continues to evolve as do the goals of therapy. Current treatment strategies include initial or early combination medical therapy with an ultimate goal of normalizing right heart function (8). In reviewing current research targets, Dr. Kim is critical of the somewhat narrow focus of available therapies. He recommends that upcoming studies not solely target the three endothelial pathways but expand to investigate interventions targeting other known or emerging pathologic pathways in PAH (9). Additionally, the lack of approved targeted therapies for the largest subgroups of pulmonary hypertension, those in WHO groups 2 and 3, represents a major clinical void and an opportunity for further investigation (10).
For the remainder of his lecture Dr. Kim outlines the past, present and future of CTEPH (11). While a history of an acute pulmonary embolus is a key risk factor for CTEPH, the disease itself is a result of more than residual clot burden. Complex processes including scar formation, inflammation, small vessel disease and right ventricular (RV) dysfunction all contribute to the pathogenesis of CTEPH (12). Furthermore, Dr. Kim emphasizes that CTEPH is not strictly an arterial disease and that patients can develop venous and capillary malformations as well. With regards to RV changes, recent studies have demonstrated that RV afterload may be greater in CTEPH when compared to other forms of pulmonary hypertension with similar degrees of pulmonary vascular resistance (13,14). Therefore, patients with CTEPH may experience relatively greater impairement of RV function than those with PAH. These findings propagate the notion that CTEPH is a unique entity of pulmonary hypertension that requires separate consideration and attention.
The scope of therapies available to patients with CTEPH remains limited but is expanding in safety, efficacy and breadth. Pulmonary thromboendarterectomy, or pulmonary endarterectomy, remains the therapy of choice for patients with CTEPH. Safety and efficacy of pulmonary thromboendarterectomy continues to improve at UCSD with in hospital mortality now below 2% (15). Furthermore, it has been found that many co-morbidities of traditional concern such as obesity, advanced age, and severity of pulmonary hypertension are not absolute contraindications for successful surgery. For those who are deemed technically inoperable due to distal chronic thromboembolic disease, Riociguat is the first and only approved medical therapy marking a major advancement in therapeutic options available to patients diagnosed with this serious condition. Riociguat has also been approved for persistent or recurrent pulmonary hypertension after pulmonary thromboendarterectomy (16). Dr. Kim emphasizes that the decision of a patient’s operability is often subjective and requires quality imaging, hemodynamics, consideration of patient factors and an experienced multidisciplinary CTEPH team.
Lastly, in addressing the future of CTEPH therapy, Dr. Kim reviews the emerging technique of balloon pulmonary angioplasty (BPA). Since early reports were published of BPA complication rates as high as 60%, centers in Japan have utilized advanced imaging modalities to refine the technique with greatly improved safety and efficacy. Through dedicated team training and cautious patient selection UCSD has successfully integrated BPA into their CTEPH treatment armamentarium. Dr. Kim emphasizes that all patients should first be evaluated and considered for pulmonary thromboendarterectomy, as this remains the only potential curative treatment. However, based on the early success of BPA procedures from Japan and across other major CTEPH centers around the world, we may see a realignment of BPA in the CTEPH treatment algorithm in the near future (17,18).
In summary, pulmonary hypertension has witnessed major advances in recent years. Not long ago, and within our generation, primary pulmonary hypertension had no effective treatment—now we have 14 FDA approved therapies with more on the horizon. CTEPH, once only treated with major cardiothoracic surgery at limited centers worldwide, now has expanded treatment options with more capable treatment centers emerging around the globe. Dr. Kim paints a picture of pulmonary hypertension as a challenging but treatable disease with a promising future.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Malhotra is PI on NIH RO1 HL085188, K24 HL132105 and co-investigator on R21 HL121794, RO1 HL 119201, RO1 HL081823. As an Officer of the American Thoracic Society, Dr. Malhotra has relinquished all outside personal income since 2012. ResMed, Inc. provided a philanthropic donation to the UC San Diego in support of a sleep center. NH Kim: Consultant—Actelion, Bayer, Merck, SteadyMed; Speaker’s bureau—Actelion, Bayer; Research support—Bellerophon, Eiger, Gilead, Lung Biotechnology; Board member—International CTEPH Association, CTEPH.com. The other author has no conflicts of interest to declare.
References
- West JB. Ibn al-Nafis, the pulmonary circulation, and the Islamic Golden Age. J Appl Physiol 1985;2008:1877-80. [PubMed]
- Newman JH. Pulmonary hypertension. Am J Respir Crit Care Med 2005;172:1072-7. [Crossref] [PubMed]
- Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med 1982;306:557-64. [Crossref] [PubMed]
- Rubin LJ, Groves BM, Reeves JT, et al. Prostacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertension. Circulation 1982;66:334-8. [Crossref] [PubMed]
- Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411-5. [Crossref] [PubMed]
- Kim NH. Pulmonary hypertension: evolution of PAH and CTEPH. American Thoracic Society Conference. Moscone Center, San Francisco, CA. May 16th, 2016. Lecture. Available online: www.thoracic.org
- Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med 2013;173:887-93. [Crossref] [PubMed]
- McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013;62:D73-81. [Crossref] [PubMed]
- Galiè N, Ghofrani AH. New horizons in pulmonary arterial hypertension therapies. Eur Respir Rev 2013;22:503-14. [Crossref] [PubMed]
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41. [Crossref] [PubMed]
- Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013;62:D92-9. [Crossref] [PubMed]
- Matthews DT, Hemnes AR. Current concepts in the pathogenesis of chronic thromboembolic pulmonary hypertension. Pulm Circ 2016;6:145-54. [Crossref] [PubMed]
- Pagnamenta A, Vanderpool R, Brimioulle S, et al. Proximal pulmonary arterial obstruction decreases the time constant of the pulmonary circulation and increases right ventricular afterload. J Appl Physiol (1985) 2013;114:1586-92. [PubMed]
- Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013;62:D22-33. [Crossref] [PubMed]
- Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012;94:97-103; discussion 103. [Crossref] [PubMed]
- Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369:319-29. [Crossref] [PubMed]
- Feinstein JA, Goldhaber SZ, Lock JE, et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001;103:10-3. [Crossref] [PubMed]
- Ogo T. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension. Curr Opin Pulm Med 2015;21:425-31. [Crossref] [PubMed]


