Transcatheter aortic valve implantation in a young heart transplant recipient crossing the traditional boundaries
Introduction
Surgical aortic valve replacement (SAVR) remains the gold standard for low and intermediate risk patients with severe valvular aortic disease. In elderly high-risk patients with symptomatic valvular heart disease, however, transcatheter aortic valve implantation (TAVI) has emerged as an alternative treatment option. A few cases of TAVI have been reported in elderly heart transplant (HTx) patients age range 75 to 81 years.
Zanuttini et al. (1) in 2013 reported a transfemoral TAVI in a high-risk 75-year-old HTx recipient (EuroSCORE 36%) 14 years after HTx. Praetere et al. (2) reported transapical TAVI in a 77-year-old high-risk HTx recipient with severely depressed left ventricular ejection fraction (LVEF) (25%), EuroSCORE 26.7% and ischemic heart disease. Seiffert et al. (3) reported a case of transapical TAVI in a high-risk 81-year-old HTx recipient (EuroSCORE of 29%) with LVEF 30%.
Case presentation
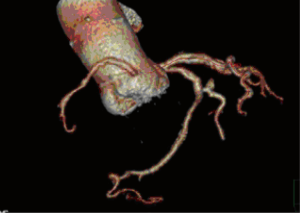
A 25-year-old woman with multiple ventricular septal defects, treated with pulmonary artery banding at the age of 1. She was re-operated with debanding of the pulmonary artery and closing of the ventricular septal defects at the age of 3. At the age of 11, she received HTx as an urgent demand due to severe congestive heart failure. The donor was a 62-year-old female. The examinations performed on the donor heart prior to HTx revealed moderate coronary arteriosclerosis, normal LVEF and mild aortic valve sclerosis. The HTx course was uneventful and the patient has been treated with statins since then. Ten years after HTx, the aortic peak gradient was 30 mmHg and after 13 years it was 60 mmHg with a moderate aortic valve insufficiency. After 14 years, she developed dyspnea (NYHA IIb). Transthoracic echocardiography demonstrated a combination of severe valvular aortic stenosis (peak gradient of 71 mmHg, mean gradient 44 mmHg and aortic valve area of <1.0 cm2) and a severe aortic valve insufficiency. Coronary angiography showed unchanged moderate coronary arteriosclerosis (Figures 1,2). Heart computed tomography (HCT) revealed massive calcification of the tricuspid aortic valve.
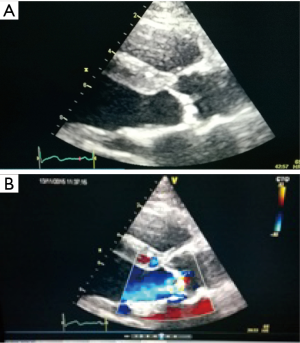
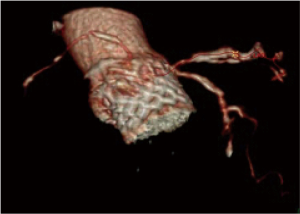
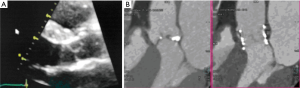
The TAVI procedure was performed according to the standard local TAVI protocol in a hybrid operating room under conscious sedation. Heparin (5,000 units) was given intra-operatively to achieve an activated clotting time (ACT) greater than 250 s. Pre-dilatation of the native valve was achieved with a 20 mm balloon. Then, a transfemoral 23 mm Edwards Sapien 3 transcatheter heart valve (Edwards Lifesciences, Irvine, CA, USA) with its delivery system was introduced within the femoral artery and advanced into the calcified native aortic valve under direct fluoroscopic control. It was positioned, guided by aortic root angiography and deployed under rapid ventricular pacing (240 beats per min). Then, percutaneous vascular closure of the access site was achieved. The procedure and the immediate post-procedural course were uneventful. The patient was discharged from the hospital on the third postoperative day. Echocardiography revealed a well positioned aortic valve prosthesis with a maximum gradient of 20 mmHg, mean gradient of 11 and normal left ventricular systolic function. At the routine 1 month follow-up the patient was doing well, without any dyspnea or physical restrain (NYHA 1). Multi-imaging with HCT, TTE and TOE echocardiography demonstrated a well positioned aortic valve prosthesis (Figures 3,4).
Discussion
The first animal TAVI was accomplished in 1989 in our institution and the first series of experiments was published in 1992. This led to TAVI application in the first human being in 2002 by Cribier et al. (4). Excellent achievements have been made in the field of TAVI during the last decade. The PARTNER trials comparing TAVI with SAVR in high-risk severe aortic stenosis patients were the milestone for TAVI (4), which showed similar survival rates at 1 year (5), improved valve hemodynamic at 2 years, reduction in symptoms, death rates, and hospitalizations (4,6). TAVI, however, showed more frequent paravalvular regurgitation and increased late mortality (4,6,7). Although TAVI has been limited to high-risk and inoperable patients, selected younger patients may also benefit this treatment strategy if individual advantages of the TAVI approach outweigh the excellent results expected from conventional SAVR (8). HTx recipients with aortic valve disease may be considered eligible candidates for TAVI because of patient-specific and procedure-specific features (3). Standard treatment for symptomatic severe aortic stenosis and/or aortic valve insufficiency in patients less than 60–65 years old is usually SAVR with a mechanical valve. Despite the young age of 25 years (but donor age of 77 years), TAVI was recommended by the heart team. It was estimated that the durability of TAVI was sufficient to allow for a later re-do HTx. As a consequence of world-wide heart donor short-ages and increased demand, marginal donors are often accepted especially in patients with an urgent call for HTx. In accord with the present case, marginal organs may increasingly be accepted because of the significant shortage of donor hearts (3,9). The marginal donor is often characterized with higher age and comorbidities such as diabetes, hypertension, left ventricular hypertrophy and even mild valvular heart disease. Survival rates after HTx has increased significantly which might increase the likelihood for development of significant age related valvular disease in the HTx population (2,3). There are no data regarding TAVI versus SAVR in HTx recipients (2).
TAVI complications as paravalvular leaks, stroke, myocardial infarction, coronary artery occlusion, aortic or annulus rupture, arrhythmias, must outweigh SAVR before low-risk patients be eligible candidates for this approach.
Conclusions
The current paper demonstrated a successful TAVI-procedure in a 25-year female 15 years after heart-transplantation. TAVI was chosen to facilitate later redo HTx.
Acknowledgements
The authors thank associate Prof. Gratien Andersen for providing 3-D pictures and video clips for this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case and accompanying images and is documented in our electronic patient journal of our department.
References
- Zanuttini D, Armellini I, Bisceglia T, et al. Transcatheter aortic valve implantation for degenerative aortic valve regurgitation long after heart transplantation. Ann Thorac Surg 2013;96:1864-6. [Crossref] [PubMed]
- De Praetere H, Ciarka A, Dubois C, et al. Transapical transcatheter aortic valve implantation in a heart transplant recipient with severely depressed left ventricular function. Interact Cardiovasc Thorac Surg 2013;16:906-8. [Crossref] [PubMed]
- Seiffert M, Meyer S, Franzen O, et al. Transcatheter aortic valve implantation in a heart transplant recipient: a case report. Transplant Proc 2010;42:4661-3. [Crossref] [PubMed]
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696-704. [Crossref] [PubMed]
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2008;29:1463-70. [Crossref] [PubMed]
- Wittwer T, Wahlers T. Marginal donor grafts in heart transplantation: lessons learned from 25 years of experience. Transpl Int 2008;21:113-25. [Crossref] [PubMed]