On the complexity of scoring acute respiratory distress syndrome: do not forget hemodynamics!
Introduction
Forecasting the outcome of patients is a daily concern. More than two thousand years ago, Hippocrates said that it is a most excellent thing for physicians to practice forecasting. Past decades have witnessed a growing interest in scores designed to predict outcome, particularly for the most critically ill patients. Scores have been developed for different goals. First, a score may help decide whether or not to perform some diagnostic procedures, as, for instance, a CT scan in suspected pulmonary embolism. Second, it should help physicians to conduct adequate randomized controlled trials in a more homogeneous population with the same risk of dying in order to test new drugs or types of management. Acute respiratory distress syndrome (ARDS), as a very heterogeneous syndrome, well illustrates that need in the light of the unlimited debates and discussions regarding the disappointing results of positive end-expiratory pressure (PEEP) trials or even prone positioning (PP) studies, until the “definitive” PROSEVA study (1). But above all, an appropriate score should allow in daily practice appropriate adjustment of therapeutic strategies in the hope of improving survival. Unfortunately, scoring systems are limited by their consubstantial link to the pattern and treatment of the development and validation cohorts. In other words, their external validity is difficult to approach.
In ARDS, a lot of studies have reported many predictors of prognosis (2-6). Very recently, Villar et al. proposed a new score called the “APPS” which is very simply based on age, PaO2/FiO2 and plateau pressure (Pplat) (7). This gives us the opportunity to reiterate how important it is to predict prognosis in ARDS, to briefly present the promising Villar et al. score, and finally to underline some forgotten parameters, focusing on hemodynamics and right ventricular (RV) function, which could help improve prognostic prediction and then management.
Outcome prediction: why and how
ARDS is still associated with a poor outcome in the intensive care unit (ICU). In the recent epidemiological cohort study from the European Society of Intensive Care Medicine in nearly 30,000 patients, ARDS accounted for 10% of ICU admissions and was associated with mortality ranging from 30% in mild cases to more than 46% in severe ones (8). After 40 years of studies seeking to understand the pathophysiology of ARDS, the 21st century started with three major therapeutic advances leading to a decrease of nearly 10% in crude mortality (1,9,10). Nevertheless, despite ventilation with a low tidal volume, the early and brief use of muscular blockade, and PP ventilation, one third to one half of patients still die (1,9,10). This is probably due in part to the disappointingly low rate of routine application of these procedures, even in the target population in which they are validated (8).
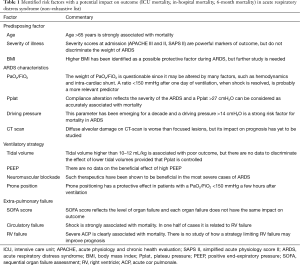
How ARDS is defined is frequently interlinked with its prognosis (Table 1). Twenty years after the landmark definition of ARDS (11), Murray et al. expanded the definition using the lung injury score (LIS) (12). A score of zero defined no lung injury, whereas a score between 0.1 and 2.5, and higher than 2.5 defined mild to moderate and severe lung injury, respectively (12). They recommended using the term ARDS only for patients with the most severe lung injury, i.e., with a score >2.5 (12). However, this score was somewhat subjective in certain regards, such as the chest X-ray evaluation, and difficult to use routinely everywhere. Moreover, Doyle et al. and Zilberberg et al. later reported that it is actually not associated with prognosis (13,14), suggesting the existence of cofounding factors between lung injury and outcome. Interestingly, Cooke et al. identified predictors of mortality very similar to those of the general intensive care population and especially confirmed that “general” severity at admission is a big marker (2). They also proposed a score combining arterial pH (protective per 0.1 more alkalotic), minute ventilation (protective when <9 L/min), PaCO2 (protective per 5 mmHg increase), and PaO2/FiO2 (associated with mortality when decreasing) (2). The overall in-hospital mortality in their study was 38.5% and the predictive value of the score was better than the acute physiology and chronic health evaluation (APACHE) III in their development cohort, although not different in their validation cohort (2). Later, the same group validated a simpler clinical predictive score (3). Age, bilirubin, fluid balance before enrollment and hematocrit were the four parameters used in the model (3). This 4-point score was elaborated in the low tidal volume group of patients included in the ARMA study (9). In the validation cohort, i.e., patients from the ALVEOLI study (15), in-hospital mortality increased from 12% for a score of zero to 67% for a score of 4. Nevertheless, this predictive score has not been validated in a nonclinical trial population and so may expose frontline intensivists to major bias.
Full table
For many years the definition of ARDS was based on the American-European consensus conference (16). In the case of bilateral infiltrates on chest X-ray without any evidence of left atrial hypertension, acute lung injury (ALI) was defined as an acute fall in PaO2/FiO2 to between 200 and 300 mmHg and as ARDS when PaO2/FiO2 is below 200 mmHg (16). Whether this definition was adapted to score the risk of death is questionable. Bersten et al. found no difference in 28-day mortality between ALI and ARDS, 32% and 34%, respectively in an Australian cohort (17). Others, as Brun-Buisson et al. in a European cohort, found a significant difference for in-hospital mortality, respectively 49.4% and 57.9% (18).
The Berlin definition has recently revisited ARDS, which is now defined as acute hypoxemia developing in a week or less, in patients with bilateral opacities on chest X-ray, having at least one risk factor for lung injury or no argument for hydrostatic edema (19). ARDS is also classified as mild, moderate or severe, according to PaO2/FiO2 following ventilation with a minimal PEEP of 5 cmH2O (19). Each stage has been demonstrated to be associated with different mortality rates, i.e., around 27–29% in mild, 32–35% in moderate and 42–45% in severe ARDS (8,19), and with different duration of mechanical ventilation in survivors (5, 7 and 9 days, respectively). However, this is still questionable, since Hernu et al. did not report that neither classification of ARDS as mild, moderate or severe nor PaO2/FiO2 is associated with mortality (20).
Very recently, following a preliminary study (4), Villar’s group proposed a simpler score for all ARDS patients, called “APPS” (7). The authors highlighted the importance of a simple, routine and reliable index of the ARDS patient’s condition that can quickly predict outcome, such as the Apgar score for newborns (21) or the Glasgow coma score for head trauma (22). This score was appropriately elaborated since it was built in a development cohort and validated in a different validation cohort. This new score is based on age, PaO2/FiO2 and Pplat separated in tertiles, 24 hours following admission (7). The minimum score is 3 and the maximum 9, corresponding respectively to 60-day survival of more than 80% and less than 20% (7).
APPS for stratifying ARDS patients
Age
Most cohort studies designed to identify mortality risk factors demonstrate a major role of age (3,5,7). Among the 414 medical patients of the low tidal volume group of the ARMA study (9), patients who died were more than 10 years older than patients who survived [60 (range, 45–72) vs. 48 (range, 37–61) years old, respectively] (3). The same result was initially reported by Villar et al. (5). Overall, age above 65 appears to be a strong predictor of mortality in ARDS. Age is linearly associated with severity in the APACHE (23,24) and also in the simplified acute physiology score II (SAPS II) (25). Based on these results, Villar et al. elaborated their score in tertiles. Patients <47 years old were attributed one point, whereas patients aged 47 to 66 and >66 were attributed two and three points, respectively (7).
Oxygenation
PaO2/FiO2 is used as a reflection of lung injury, yet many factors that have nothing to do with the severity of lung injury may affect blood gas analysis. Hemodynamic failure, which will be discussed further below, may especially contribute to PaO2/FiO2 by two mechanisms that have opposite effects. The first, called low PvO2 effect, leads to a decrease in oxygenation and then to an overestimation of lung injury. Lemaire et al. demonstrated that, for a given shunt fraction, PaO2/FiO2 dramatically decreases in patients with an elevated arteriovenous oxygen content difference (due to an increase in oxygen extraction) compared with the others (26). The second mechanism regards alterations of shunt fraction according to cardiac output changes. A low cardiac output decreases the shunt, increases PaO2/FiO2 and so may lead to underestimation of lung injury (27). Another mechanism may also limit interpretation of PaO2/FiO2 as a marker of lung injury. This is intra-cardiac shunt through a patent foramen ovale because the right ventricle is overloaded. Mekontso Dessap et al. reported that it occurs in close to 20% of ARDS patients submitted to protective ventilation and is associated with a significant increase in duration of ventilation (28). Pulmonary imaging as well as respiratory mechanics may reflect lung injury more efficiently. Rouby et al. reported that differences in lung morphology evaluated by CT-scan and in respiratory mechanics help identify ARDS with a high mortality, since mortality was 75% in patients with diffuse attenuations and 42% in patients with lobar attenuations (29). Finally, PaO2/FiO2 is strongly related to respiratory settings. In the ARMA study, patients ventilated with 12 mL/kg had better PaO2/FiO2 ratios than patients ventilated with low tidal volume, but a poorer outcome (9). In the Berlin consensus conference, experts proposed standardizing ventilation with a PEEP of 5 cmH2O and a tidal volume of 6 mL/kg to so as to interpret PaO2/FiO2 correctly (9). However, as shown by the recent ESICM trial, many intensivists still do not ventilate patients with such a low tidal volume (8). In the preliminary study by Villar’s group, PaO2/FiO2 did not differ significantly between survivors and non-survivors (115±41 vs. 106±39, respectively, P=0.054), but the overall population actually had severe ARDS (4). Hernu et al. also reported no difference in mortality according to the PaO2/FiO2 ratio (20). Nevertheless, Cooke et al. demonstrated an association between death and the lowest PaO2/FiO2 ratio (3). In their APPS, Villar et al. constructed tertiles, in which patients with a PaO2/FiO2 <158 mmHg were given two points and those with a ratio <105 mmHg were given three points (7). These tertiles appear to be in accordance with studies demonstrating the beneficial effects of neuromuscular blockade and PP for patients with a PaO2/FiO2 below 150 mmHg (1,10).
Pplat
The third parameter of the APPS focuses on respiratory mechanics. Pplat is the consequence of tidal volume and compliance of the respiratory system and may be understood as a surrogate of lung stress/transpulmonary pressure at end-inspiration. It is now widely established that ARDS is characterized by diffuse alveolar damage (30), which can be dramatically worsened by ventilator-induced lung injury (VILI) (31-33). Since the beginning of the 1990s, authors have demonstrated the value of limiting tidal volume and Pplat in reducing mortality (9,34). More recently, Amato et al. as well as Bellani et al. provided some clarification by showing that the deleterious effects of tidal ventilation are actually more related to the driving pressure (Pplat minus total PEEP), reflecting the lung stress induced by inspiration (8,35,36). For “APPS”, Pplat is separated in tertiles: <27 cmH2O (one point), 27–30 cmH2O (two points) and >30 cmH2O (three points) (7).
The “forgotten” hemodynamics
ARDS is very frequently associated with hemodynamic failure, since more than 60% of patients have shock (37) and 65% require infusion of catecholamines (38,39). As discussed above, hemodynamic failure limits the accuracy of PaO2/FiO2 in evaluating the severity of lung injury. In most of the predictors previously discussed, hemodynamics per se is not evaluated. Yet, Doyle et al. reported that in-hospital mortality is very similar (56% vs. 59%) in patients with a PaO2/FiO2 < or >150 mmHg, whereas nonpulmonary organ system dysfunction is a strong predictor of mortality (13). Vieillard-Baron et al. reported in 98 patients with moderate to severe ARDS that septic shock or the need for epinephrine/norepinephrine infusion is strongly associated with mortality, although the level of hypoxemia is not, suggesting that hemodynamic failure is key among nonpulmonary organ dysfunctions (40). Later, Page et al. reported parameters associated with mortality in 150 patients with moderate to severe ARDS (41). The overall hospital mortality was 38%. The only two parameters independently associated with mortality were PaO2/FiO2, with an odds ratio (OR) of 1.01 (1.00–1.02), and the severity of circulatory failure, with an OR of 10.17 (3.43–30.32), whereas LIS was not (41). In a cohort of more than 752 patients with moderate to severe ARDS submitted to protective ventilation, Mekontso Dessap et al. recently re-emphasized that shock is one of the factors independently associated with mortality and has the highest OR [3.25 (2.32–4.56)] among age, SAPS II, and a PaO2/FiO2 <100 mmHg during the first 2 days following mechanical ventilation [OR 1.45 (1.02–2.08)] (37). In these two last studies, PaO2/FiO2 was slightly but significantly associated with mortality. However, it was recorded a few hours or days following mechanical ventilation in a period when hemodynamics is stabilized and may therefore reflect severity more accurately. This is the position of Villar et al., who record PaO2/FiO2 24 hours after ventilation.
Apart from sepsis, which is frequently associated and responsible for shock in half of ARDS patients (18), one of the main causes of hemodynamic failure is RV failure. The reasons for such RV failure are many, and all lead to acute pulmonary hypertension and increased RV afterload: lung inflammation induces pulmonary vascular injury (42), hypoxemia and respiratory acidosis induce pulmonary vasoconstriction and mechanical ventilation may induce a vascular waterfall at the level of the pulmonary capillaries (43). In this situation, RV failure is detected by echocardiography as a pattern of acute cor pulmonale (ACP), which associates RV dilatation and paradoxical septal motion, without significant RV hypertrophy (44). Risk factors for ACP are pneumonia-related ARDS, PaO2/FiO2 <150 mmHg, driving pressure ≥18 cmH2O and PaCO2 ≥48 mmHg, with an incidence of ACP less than 10% in the absence of risk factors, but of 60% when four risk factors are present (37). Severe ACP (the right ventricle is bigger than the left) has been reported as independently associated with mortality with an OR of 1.89 (1.08–3.30) (37). Interestingly, it has been reported that PP may normalize RV function (45) and also improve hemodynamics (46). On the other hand, the beneficial effect of PP on survival is not related to blood gas analysis changes (47), whereas PP increases the number of cardiovascular failure-free days up to 28 days after randomization and decreases the incidence of cardiac arrest (1). In the development and validation cohorts of the APPS, it seems that patients did not benefit from PP (at least it is not reported), which could result in a major bias (7).
Conclusions
Scoring the outcome of patients suffering from ARDS is one of the most difficult evaluations that intensivists have to face, because the “salad” of ARDS mixes cabbages and carrots. Heterogeneity regards patients, lung injury and histologic lesions, but also treatments, some of which are proven to change the prognosis. This could explain the huge variability among studies. However, it is clear that potential circulatory and RV failures play a great role and should be taken into account so as to classify patients well, since ARDS is most certainly not a single-organ failure. The “geocentric” vision of ARDS focused on the lung and gas exchange must be replaced by a more post-Copernican and “heliocentric” vision including hemodynamics and especially the RV, on the one hand, and genetic variants on the other.
Acknowledgements
Support was provided solely from institutional and/or departmental sources.
Footnote
Provenance: This is an invited Perspective commissioned by the Section Editor Zhongheng Zhang (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Cooke CR, Kahn JM, Caldwell E, et al. Predictors of hospital mortality in a population-based cohort of patients with acute lung injury. Crit Care Med 2008;36:1412-20. [Crossref] [PubMed]
- Cooke CR, Shah CV, Gallop R, et al. A simple clinical predictive index for objective estimates of mortality in acute lung injury. Crit Care Med 2009;37:1913-20. [Crossref] [PubMed]
- Villar J, Fernández RL, Ambrós A, et al. A clinical classification of the acute respiratory distress syndrome for predicting outcome and guiding medical therapy*. Crit Care Med 2015;43:346-53. [Crossref] [PubMed]
- Villar J, Pérez-Méndez L, Basaldúa S, et al. A risk tertiles model for predicting mortality in patients with acute respiratory distress syndrome: age, plateau pressure, and P(aO(2))/F(IO(2)) at ARDS onset can predict mortality. Respir Care 2011;56:420-8. [Crossref] [PubMed]
- Ware LB, Koyama T, Billheimer DD, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest 2010;137:288-96. [Crossref] [PubMed]
- Villar J, Ambrós A, Soler JA, et al. Age, PaO2/FiO2, and Plateau Pressure Score: A Proposal for a Simple Outcome Score in Patients With the Acute Respiratory Distress Syndrome. Crit Care Med 2016;44:1361-9. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988;138:720-3. [Crossref] [PubMed]
- Doyle RL, Szaflarski N, Modin GW, et al. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med 1995;152:1818-24. [Crossref] [PubMed]
- Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med 1998;157:1159-64. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- Bersten AD, Edibam C, Hunt T, et al. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med 2002;165:443-8. [Crossref] [PubMed]
- Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 2004;30:51-61. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Hernu R, Wallet F, Thiollière F, et al. An attempt to validate the modification of the American-European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Med 2013;39:2161-70. [Crossref] [PubMed]
- Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 1953;32:260-7. [Crossref] [PubMed]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81-4. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Zimmerman JE, Kramer AA, McNair DS, et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med 2006;34:1297-310. [Crossref] [PubMed]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63. [Crossref] [PubMed]
- Lemaire F, Jardin F, Regnier B, et al. Pulmonary gas exchange during venoarterial bypass with a membrane lung for acute respiratory failure. J Thorac Cardiovasc Surg 1978;75:839-46. [PubMed]
- Dantzker DR, Lynch JP, Weg JG. Depression of cardiac output is a mechanism of shunt reduction in the therapy of acute respiratory failure. Chest 1980;77:636-42. [Crossref] [PubMed]
- Mekontso Dessap A, Boissier F, Leon R, et al. Prevalence and prognosis of shunting across patent foramen ovale during acute respiratory distress syndrome. Crit Care Med 2010;38:1786-92. [Crossref] [PubMed]
- Rouby JJ, Puybasset L, Cluzel P, et al. Regional distribution of gas and tissue in acute respiratory distress syndrome. II. Physiological correlations and definition of an ARDS Severity Score. CT Scan ARDS Study Group. Intensive Care Med 2000;26:1046-56. [Crossref] [PubMed]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [Crossref] [PubMed]
- Dreyfuss D, Soler P, Basset G, et al. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 1988;137:1159-64. [Crossref] [PubMed]
- Hickling KG. Ventilatory management of ARDS: can it affect the outcome? Intensive Care Med 1990;16:219-26. [Crossref] [PubMed]
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 1990;16:372-7. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Costa EL, Slutsky AS, Amato MB. Driving pressure as a key ventilation variable. N Engl J Med 2015;372:2072. [PubMed]
- Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862-70. [Crossref] [PubMed]
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. [Crossref] [PubMed]
- McAuley DF, Laffey JG, O'Kane CM, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med 2014;371:1695-703. [Crossref] [PubMed]
- Vieillard-Baron A, Girou E, Valente E, et al. Predictors of mortality in acute respiratory distress syndrome. Focus On the role of right heart catheterization. Am J Respir Crit Care Med 2000;161:1597-601. [PubMed]
- Page B, Vieillard-Baron A, Beauchet A, et al. Low stretch ventilation strategy in acute respiratory distress syndrome: eight years of clinical experience in a single center. Crit Care Med 2003;31:765-9. [Crossref] [PubMed]
- Zapol WM, Kobayashi K, Snider MT, et al. Vascular obstruction causes pulmonary hypertension in severe acute respiratory failure. Chest 1977;71:306-7. [Crossref] [PubMed]
- Fessler HE, Brower RG, Shapiro EP, et al. Effects of positive end-expiratory pressure and body position on pressure in the thoracic great veins. Am Rev Respir Dis 1993;148:1657-64. [Crossref] [PubMed]
- Vieillard-Baron A, Prin S, Chergui K, et al. Echo-Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med 2002;166:1310-9. [Crossref] [PubMed]
- Vieillard-Baron A, Charron C, Caille V, et al. Prone positioning unloads the right ventricle in severe ARDS. Chest 2007;132:1440-6. [Crossref] [PubMed]
- Teboul JL, Zapol WM, Brun-Buisson C, et al. A comparison of pulmonary artery occlusion pressure and left ventricular end-diastolic pressure during mechanical ventilation with PEEP in patients with severe ARDS. Anesthesiology 1989;70:261-6. [Crossref] [PubMed]
- Albert RK, Keniston A, Baboi L, et al. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med 2014;189:494-6. [Crossref] [PubMed]


