Surgical outcomes associated with postoperative atrial fibrillation after robotic-assisted pulmonary lobectomy: retrospective review of 208 consecutive cases
Introduction
Atrial fibrillation is a frequent postoperative complication after non-cardiac thoracic surgery. Iwata and colleagues found that the incidence of post-operative atrial fibrillation (POAF) lies between 3.1–28% in non-cardiac thoracic surgery patients after analysis of 24 studies (1). Unlike non-operative related atrial fibrillation, POAF is not without consequences. It has been reported that POAF after thoracic surgery is associated with increased mortality rate, hospital length of stay (LOS), and cost of hospital stay (2), even in patients with POAF as the sole postoperative complication (3). Not only is POAF associated with poorer perioperative outcomes and hospital mortality, it has also been linked to reduced long term survival after surgery (4). The incidence of and complications associated with POAF implied that more studies and evidence were needed to further evaluate the pathophysiology and eventually the management regimen needed to improve hospital course and perioperative outcome in non-cardiac thoracic surgery.
Meanwhile, the use of robotic-assisted videothoracoscopic surgery (RAVTS) lobectomy has been steadily rising. However, lack of long-term data has precluded studies to compare long-term outcomes between conventional video-assisted thoracoscopic surgery (VATS) and RAVTS lobectomy. In terms of short-term outcomes, mixed results have been reported by different studies. For example, Paul et al. found that, in early experiences with robotic-assisted surgery, RAVTS was associated with higher rates of intraoperative injury and bleeding than did conventional VATS (5). However, Jang et al. associated RAVTS with less intraoperative blood loss, fewer postoperative complications, and shorter hospital LOS than VATS in their study (6). In order to fully evaluate the pros and cons of robotic assistance during lung resection, more studies are needed to evaluate significant postoperative complications and hospital course.
To our best knowledge, no study has been conducted to analyze POAF after RAVTS lobectomy, despite Velez-Cubian et al. having shown that POAF is indeed among the five most common postoperative complications, with atelectasis or mucous plugging, prolonged air leak >7 days, acute respiratory distress syndrome or respiratory failure, and pneumonia after review of 23 series of RAVTS series (7). Therefore, this study was conducted to investigate the perioperative outcomes, such as hospital course, chest tube duration, and in-hospital mortality associated with POAF after robotic-assisted thoracoscopic lobectomy.
Methods
We performed a retrospective analysis of prospectively collected data from 211 consecutive patients who underwent robotic-assisted pulmonary lobectomy by a single surgeon over a 33-month period from September 2010 through May 2013 at our institution.
The date for this study was obtained through a Thoracic Oncology Program Clinical Research Database protocol approved by our institution’s Scientific Review Committee (MCC#16512) and our university’s Institutional Review Board (IRB #Pro00002678). Informed consent for this retrospective review of existing data was waived by the IRB. However, all of our patients gave informed consent for our standard surgical procedure that consisted of fiberoptic bronchoscopy, robotic-assisted video-thoracoscopic wedge resection and/or robotic-assisted video-thoracoscopic (completion) lobectomy, mediastinal lymph node dissection (MLND), with possible thoracotomy, and also gave permission to use surgical data for educational and research purpose.
All our patients undergo fiberoptic bronchoscopy by the operating surgeon after the induction of general anesthesia. After placement of the dual-lumen endotracheal tube, the patient is then placed in either right or left lateral decubitus position, depending on which hemithorax the lesion is located. Our robotic-assisted lobectomy technique utilizes a three-port system, which includes a 4-cm camera port along the 6th intercostal space (ICS) at the anterior axillary line, which doubles as the assistant’s access port, and two 1-cm instrument ports along the 3rd ICS at the anterior axillary line and along the 9th ICS at the posterior axillary line. From September 2010 through December 2011, our group used the da Vinci® (Intuitive Surgical Corporation, Sunnyvale, CA, USA) “S”™ robotic surgical system, with the “Si”™ system being used from January 2012 to the present. Lobectomy is performed with the pulmonary vein divided first, then division of the pulmonary artery branch(es) and bronchus, and then completion of the pulmonary fissures. After delivery of the lobectomy within an endopouch through the 6th ICS incision, complete MLND is then performed. At the end of the procedure, a 32-French chest tube is introduced through the 9th ICS incision and connected to drainage at −20 cm H2O continuous suction.
Three patients out of 211 were converted to open pneumonectomy and excluded from this study. We investigated the difference in surgical outcomes by measuring parameters including post-operative complications, chest tube duration, and hospital LOS between patients who experienced POAF within 30 days after surgery and those who did not.
Clinically significant postoperative complications other than POAF were recorded, including pulmonary-related complications, such as pulmonary embolism, respiratory failure, hemothorax, chyle leak, pneumothorax or mucus plug that required intervention, prolonged air leak that lasted for more than 7 days, and aspiration incidents, as well as cardiac arrhythmia other than POAF and anemia that required transfusion. Intraoperative complications, such as bleeding from pulmonary and other vessels and injury to the phrenic or recurrent laryngeal nerve, airway, or diaphragm, were also recorded. POAF was detected by continuous telemetry monitoring from time of surgery until hospital discharge on all surgical patients after lobectomy.
Extensive literature reviews were then performed on surgical outcomes published for case series after thoracotomy or conventional VATS. However, the little available data about POAF after robotic-assisted surgery prompted our study to address the association between POAF and perioperative outcomes after robotic-assisted surgery.
We report our results as mean or median, standard error of the mean (SEM), and range, unless otherwise specified. We used χ2, Fisher’s exact test, or Student’s t-test to compare the two groups of patient. Statistical significance was determined at P≤0.05.
Results
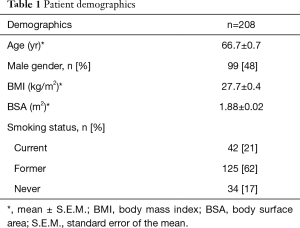
Among the 208 patients in the study, the median age of our entire patient cohort was 66.7±0.7 yr. Of all 208 patients, 99 (48%) of them were male. The average body mass index (BMI) and total body surface area (BSA) of the entire patient cohort studied were 27.7±0.4 kg/m2 and 1.88±0.02 m2, respectively. In regards to the patients’ smoking status, 21% of them were current smokers or quit less than 6 months prior to surgery, 62% of them were former smokers who quit more than 6 months prior to surgery, and 17% of them had never smoked. Patients’ demographic characteristics are summarized in Table 1.
Full table
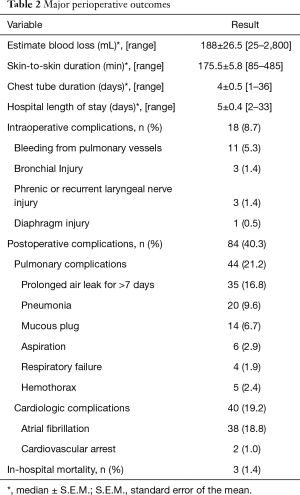
Combining all 208 cases, the median estimated blood loss (EBL) is 187.5±26.5 mL. The median skin-to-skin operative time is 175.5±5.8 min. Our patients’ median chest tube duration and hospital LOS were 4±0.5 and 5±0.4 days, respectively. Overall intraoperative complication and postoperative complication rates were 8.7% and 40.3%, respectively. Three in-hospital mortalities were documented in the first 40 cases of the study period, which made up 1.9% of the entire 208 cases studied. Overall intraoperative and postoperative outcomes from all 208 patients are summarized in Table 2.
Full table
Out of 208 patients reviewed, 19% (39 patients) developed POAF and were compared to the 81% of patients who did not. The median onset of POAF was on postoperative day 2. Nine out of 39 patients with POAF had onset on or after postoperative day 4, which was the median hospital LOS of patients without POAF. Out of 39 patients who developed POAF, 12 patients had previously documented atrial fibrillation prior to the surgery. Therefore, new-onset POAF occurred in 27 patients, which comprised 13.0% of all 208 cases.
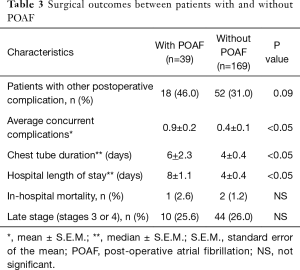
Forty-six percent (18 out of 39) of patients with POAF developed other concurrent postoperative complications, although this percentage did not differ statistically from the percentage (31%) in patients without POAF. However, patients who experienced POAF had a significantly higher mean number of concurrent complications, which was calculated by dividing the total number of postoperative complications by the number of patients in that group, compared to the patients without POAF. The group of patients with POAF experienced an average of 0.9 complications per patient, compared to an average of 0.4 complications per patient in the group without POAF.
Compared to the patients without POAF, who had median chest tube duration and median hospital LOS that were both 4 days, patients who developed POAF had significantly longer median chest tube duration and median hospital LOS, which were 6 and 8 days, respectively. Despite the longer chest tube duration and hospital LOS, no significant difference in mortality between the two groups was shown in this study. Table 3 summarizes the differences in postoperative outcomes between patients who developed POAF and those who did not.
Full table
Discussion
The perioperative outcomes in our study are comparable to currently available literature on robotic–assisted pulmonary lobectomy. Nakamura (8) analyzed nine series of RAVTS lobectomies and pooled 693 cases total. In his analysis, the average operative time and conversion rate to open cases were 188 min and 8.8%, respectively. The pooled average hospital LOS and mortality were 5.2 days and 0.8%, respectively. Compared to his analysis and another literature review of 6 RAVTS lobectomy series, with an average operative time of 215 min and hospital LOS of 6 days (9), our series, with median operative time of 175 min, median hospital LOS of 5 days, and in-house mortality of 1.4%, had similar perioperative outcomes.
Perioperative outcomes after conventional VATS, from the same analysis conducted by Nakamura, reported on average 4.1 hr of surgery time, 123.7 mL of EBL, 4 days of chest tube duration, and 13.2 days of hospital LOS (8). That same study showed that VATS had shorter chest tube duration and hospital LOS than did open lobectomy, which were 5.5 and 16.2 days, respectively, despite a longer surgery time with VATS. In our study, our series had the same chest tube duration (4 days) compared with VATS analyzed in that Nakamura study, but with a much shorter hospital LOS (5 days).
As mentioned earlier, POAF rates have been reported to be between 3.1–28% in non-cardiac thoracic surgery patients (1). However, this high variability depends on the type of surgery (esophageal surgery vs. lung surgery), degree of resection (wedge vs. lobectomy vs. pneumonectomy), type of monitoring [multiple electrocardiograms (ECG) vs. continuous telemetry], inclusion of preoperative atrial fibrillation patients, and so forth. One of the studies had a POAF rate of 13% and 21% in VATS and open lobectomy, respectively (10). From our literature review, POAF was frequently documented as the most common postoperative complication after RAVTS lobectomy and varied between 4–13% in six different series (11-16).
Despite comparable outcomes and shorter hospital LOS after RAVTS in our series, our POAF rate was 18.8%, which is slightly higher than other RAVTS lobectomy series we found on literature review, but within the ranges we found among other non-cardiac thoracic surgeries. However, after we exclude the 12 patients with atrial fibrillation prior to surgery, the incidence of new-onset POAF in our series was 13.0%, which is not inferior to most of the RAVTS series we reviewed in the literature. Moreover, the incidence of POAF in this study is likely higher than that in prior studies due to the use of continuous telemetry until hospital discharge for patients who underwent robotic-assisted lobectomy, which allows us to identify more POAF events during their hospital course compared to single or multiple ECGs, which would be expected to miss brief POAF events. For example, patients who had their vital signs measured only every 4 hours could have had POAF events that lasted less than 4 hours and that could have remained undocumented. In addition, a longer median hospital LOS was noted in patients with POAF, which may have also skewed their POAF rate when there was another complication that prolonged their hospital LOS and that allowed identification of POAF events occurring after 4 days, the median hospital LOS for the patients without POAF, which happened to nine patients with POAF in this study.
All patients who underwent lobectomy, regardless of stage, were included in this study, instead of only early stage lung cancer, as is usual in the other series. Although the stage of pulmonary malignancy was identified as one of the risk factor for POAF by both univariate and multivariate analysis (17), no significant difference of percentage of late stage disease (stage 3 and 4) is found in our series between patients with and without POAF, which is 25.6% and 26%, respectively.
The relatively inclusive POAF incidence captured in our series, compared to published rates for VATS and other RAVTS series, permitted a statistical investigation of the association of POAF and surgical outcomes. This current series showed that patients who experienced POAF had significantly longer chest tube duration and hospital LOS. The association between POAF and prolonged hospital LOS demonstrated in this series was similar to results from other studies on open or VATS approaches for pulmonary resection (18,19). In a study done after lung resection via thoracotomy, Roselli et al. reported a longer median hospital LOS (8 days) in patients with POAF that required treatment than in patients without POAF (5 days), even with a higher incidence of POAF despite a more rigorous definition of POAF (19). It is unlikely that the association between POAF and prolonged hospital LOS was strictly causative. The longer hospital LOS could possibly have been caused by POAF directly or by the possibility that POAF occurred in already sicker and more complicated patients. The latter explanation can be supported by the significantly longer chest tube duration and higher number of concurrent complications per patient observed postoperatively in the POAF group in this study. Despite being not significant, the percentage of patients with concurrent complications in the group of patients with POAF appears to be higher than the percentage in the group of patients without POAF (46% vs. 31%). No in-house mortality difference was found between the two groups in this study.
However, the association between POAF and perioperative complications of surgical patients may not be as obvious as stated, and results have been mixed. A recent study comparing VATS lobectomy and open lobectomy found that, despite the significantly lower numbers of postoperative complications, major cardiopulmonary complications, including pneumonia, prolonged air leak, atelectasis requiring bronchoscopy, and acute respiratory distress, and shorter hospital LOS in VATS lobectomy cases, no significant difference were observed in POAF between open lobectomy and VATS (20). Park et al. also found no significant reduction of POAF incidence (12%) after VATS compared to that after open lobectomy (16%), despite its outcome benefits of shorter hospital LOS and fewer complications, after studying 244 matched patients (21). However, it is also possible that either paper did not have a large enough sample size or power to determine a significant difference in POAF incidence between VATS and open cases. Papiashvilli et al. showed that VATS reduced the incidence of postoperative complications, including POAF, compared to open lobectomy (22). Ivanovic et al. also showed a significant reduction of POAF incidence in thoracoscopic lung resection compared to thoracotomy (17).
The results of the current study suggested that POAF affects robotic-assisted lobectomy outcomes as much as outcomes after thoracotomy and VATS. Both the medical and economic burdens implicate the importance of the incidence of POAF on lobectomy patients. Risk factors and intraoperative ways to reduce POAF incidence should be further investigated after robotic-assisted lobectomy.
Whether POAF is the cause of or a cofounding factor to its association with worse surgical outcomes after pulmonary anatomical resection, its negative impact on patients’ perioperative outcome is undeniable. Moreover, it is associated with poorer long-term survival (10% vs. 57%) of malignancy at 17 months after lobectomy (23). This present study confirmed the results in prior literature on the relatively new surgical technique of robotic-assisted lobectomy. The mechanism and etiology of POAF is currently unclear. Therefore, more studies are needed to determine the effect and etiology of POAF after lobectomy with different surgical approaches (open vs. VATS vs. RAVTS).
Conclusions
Our perioperative outcomes with robotic-assisted lobectomy are comparable to those in the literature that we reviewed for robotic-assisted lung surgery. The efficacy and safety of robotic-assisted lung surgery appears to be similar to those of VATS and open lobectomy. The rate of POAF in the present series was within the range of POAF rates after non-cardiac thoracic surgery in the literature and in other series of RAVTS. Although our patients had an overall POAF rate of 18.8%, our rate of new-onset POAF is 13% after excluding patients with atrial fibrillation prior to surgery and is similar to that published for VATS (13%) and better than that for open lobectomy (21%) (10). However, patients who experienced POAF in this study were associated with significantly more postoperative complications per patient, longer chest tube duration, and longer hospital LOS, with no significant difference in in-house mortality, compared to patients without POAF. The finding of poorer outcomes in POAF patients is similar to those of studies we found in the literature regarding POAF patients after VATS lobectomy and open lobectomy. Regardless of surgical approach, POAF poses a medical and economic burden after lobectomy. Further evidence is needed to study the risk factors and intraoperative outcomes affecting the incidence of POAF.
Acknowledgements
This research was supported by a 2013 Summer Scholarly Award to KL Rodriguez and a 2014 Summer Scholarly Award to EP Ng from the Scholarly Concentrations Program at the University of South Florida (USF) Health Morsani College of Medicine and by financial support to MR Thau from the Scholarly Excellence, Leadership Experiences, and Collaborative Training (SELECT) Program of the USF Health Morsani College of Medicine and the Lehigh Valley Health Network.
Footnote
Conflicts of Interest: EM Toloza and JP Fontaine have financial relationships with Intuitive Surgical Corporation in form of honoraria as robotic thoracic surgery proctors and observation sites. This study was previously presented as a Poster at the 6th Latin American Conference on Lung Cancer in Lima, Peru, on August 23, 2014, and as an Oral Presentation at the Annual Academic Surgical Congress 2015 in Las Vegas, NV, on February 5, 2015.
Ethical Statement: The data for this study was obtained through a Thoracic Oncology Program Clinical Research Database protocol approved by our institution’s Scientific Review Committee (MCC#16512) and our university’s Institutional Review Board (IRB #Pro00002678). Informed consent for this retrospective review of existing data was waived by the IRB.
References
- Iwata T, Nagato K, Nakajima T, et al. Risk factors predictive of atrial fibrillation after lung cancer surgery. Surg Today 2016;46:877-86. g. [Crossref] [PubMed]
- Bhave PD, Goldman LE, Vittinghoff E, et al. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J 2012;164:918-24. [Crossref] [PubMed]
- Vaporciyan AA, Correa AM, Rice DC, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg 2004;127:779-86. [Crossref] [PubMed]
- Amar D, Burt M, Reinsel RA, et al. Relationship of early postoperative dysrhythmias and long-term outcome after resection of non-small cell lung cancer. Chest 1996;110:437-9. [Crossref] [PubMed]
- Paul S, Jalbert J, Isaacs AJ, et al. Comparative effectiveness of robotic-assisted vs thoracoscopic lobectomy. Chest 2014;146:1505-12. [Crossref] [PubMed]
- Jang HJ, Lee HS, Park SY, et al. Comparison of the early robot-assisted lobectomy experience to video-assisted thoracic surgery lobectomy for lung cancer: a single-institution case series matching study. Innovations (Phila) 2011;6:305-10. [Crossref] [PubMed]
- Velez-Cubian FO, Ng EP, Fontaine JP, et al. Robotic-Assisted Videothoracoscopic Surgery of the Lung. Cancer Control 2015;22:314-25. [PubMed]
- Nakamura H. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2014;20:93-8. [Crossref] [PubMed]
- Takagi H, Yamamoto H, Goto SN, et al. Perioperative results of robotic lung lobectomy: summary of the literature. Surg Endosc 2012;26:3697-9. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Augustin F, Bodner J, Wykypiel H, et al. Initial experience with robotic lung lobectomy: report of two different approaches. Surg Endosc 2011;25:108-13. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg 2008;85:1880-5; discussion 1885-6.
- Jett GK. Thoracic robotics at the Heart Hospital Baylor Plano: the first 20 cases. Proc (Bayl Univ Med Cent) 2012;25:324-6. [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Toker A. Robotic thoracic surgery: from the perspectives of European chest surgeons. J Thorac Dis 2014;6 Suppl 2:S211-6. [PubMed]
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Ivanovic J, Maziak DE, Ramzan S, et al. Incidence, severity and perioperative risk factors for atrial fibrillation following pulmonary resection. Interact Cardiovasc Thorac Surg 2014;18:340-6. [Crossref] [PubMed]
- Cardinale D, Martinoni A, Cipolla CM, et al. Atrial fibrillation after operation for lung cancer: clinical and prognostic significance. Ann Thorac Surg 1999;68:1827-31. [Crossref] [PubMed]
- Roselli EE, Murthy SC, Rice TW, et al. Atrial fibrillation complicating lung cancer resection. J Thorac Cardiovasc Surg 2005;130:438-44. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Park BJ, Zhang H, Rusch VW, et al. Video-assisted thoracic surgery does not reduce the incidence of postoperative atrial fibrillation after pulmonary lobectomy. J Thorac Cardiovasc Surg 2007;133:775-9. [Crossref] [PubMed]
- Papiashvilli M, Stav D, Cyjon A, et al. Lobectomy for non-small cell lung cancer: differences in morbidity and mortality between thoracotomy and thoracoscopy. Innovations (Phila) 2012;7:15-22. [Crossref] [PubMed]
- Imperatori A, Mariscalco G, Riganti G, et al. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg 2012;7:4. [Crossref] [PubMed]


