Serum selenium levels in patients with respiratory diseases: a prospective observational study
Introduction
In up to 10–50% of intensive care unit (ICU) patients, sepsis and systemic inflammatory responses are mainly responsible for increasing the mortality. Despite advancements in critical care medicine over several years, there has been no significant decrease in the mortality (1).
Oxidative stress arises from an imbalance between reactive oxygen species and anti-oxidants. The increased oxidative stress is associated with a poor prognosis in ICU patients. This leads to the increase in systemic inflammatory responses and oxidative stress in an ICU setting. Moreover, there is a growing interest in the effects of antioxidants such as omega-3 fatty acids and gamma-linolenic acid in preventing the worsening of the status of ICU patients (2-4).
Selenium is a trace element for humans, and it plays a role as an antioxidant in acute stress conditions such as systemic inflammatory responses or trauma. Thus, it is involved in protecting cells from oxidative stress (5). In 1990, Hawker et al. first reported that serum selenium levels were significantly lower in ICU patients as compared with normal healthy controls (6). Since then, some studies have reported that they were relatively lower in patients with severe sepsis or septic shock who were admitted to a medical and surgical ICU (7,8). Still, however, controversial opinions exist regarding the relationship between serum selenium levels and the prognosis in ICU patients.
There may be a variability in serum selenium levels depending on soils and dietary habit. This leads to the speculation that individuals with a residency in areas with an insufficient amount of selenium or those with a decreased selenium intake due to different dietary habits are at risks of developing low serum selenium levels (9,10). Still, however, there is a paucity of data regarding not only whether there is any difference in serum selenium levels depending on the severity of respiratory diseases between the ICU and general wards in hospitalized patients with non-traumatic or non-surgical changes but also whether such changes are associated with inflammatory markers and prognostic factors particularly including nutritional factors in Asian countries with differences in soils and dietary habits from western ones.
Given the above background, we conducted this study to examine the difference in serum selenium levels according to the severity of respiratory diseases between the patients admitted to the ICU and general wards. In addition, we also attempted to identify the factors associated with low serum selenium levels in critically ill patients with respiratory diseases.
Methods
Study patients and setting
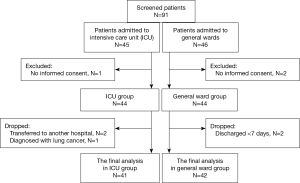
We enrolled a consecutive series of 91 patients with respiratory diseases who had admitted to the ICU or general wards of the respiratory center of a university hospital from August 2011 until July 2012. We divided our clinical series of patients into either of two groups, the ICU group or the general ward group, based on the following eligibility criteria. Inclusion criteria are as follows: (I) patients aged 19 years or older; (II) patients who visited an emergency room or the outpatient clinic of our medical institution for further evaluation and treatment of acute infectious or inflammatory respiratory diseases or worsening of chronic ones [e.g., pneumonia, asthma, chronic obstructive pulmonary disease (COPD), adult respiratory distress syndrome (ARDS), tuberculosis or pneumoconiosis]. Exclusion criteria for the study are as follows: (I) patients with a past history of malignancy or diagnosed malignancies; (II) pregnant women; (III) patients who were referred from other hospitals.
All the patients with systemic inflammatory response syndrome (SIRS) and PaO2/FiO2 ratio of ≤300 mmHg were enrolled in the ICU group. A diagnosis of SIRS was made in the patients who met more than one criteria of the American College of Chest Physicians/Society of Critical Care Medicine, i.e., body temperature of >38 or <36 °C, heart rate of >90 beats/min, respiratory rate of >20 breaths/min or hyperventilation with PaCO2 <32 mmHg, white blood cell (WBC) counts of >1.2×103/mm3, <4.0×103/mm3, or immature WBC counts of >10%. The study was approved by the Institutional Review Board of our medical institution (approval No. 2010-15).
Sample size estimation
To estimate the sample size, we considered 5% type I error rate for a two sided test and 20% type II error rate, respectively. The expected correlation coefficient was set 0.3 and then the sample size was estimated 85. We aimed to assign 44 patients to each group considering the possibility that they might be dropped out during a one-week follow-up period. Of the 45 patients of the ICU group, one did not submit a written informed consent. In addition, of the 46 patients of the general ward group, two did not submit a written informed consent. We therefore enrolled a total of 88 patients who submitted a written informed consent.
Disposition of the study patients
During the one-week follow up, three patients of the ICU group were dropped out of the study (two were transferred to another hospital and one was diagnosed with lung cancer) and two patients of the general ward group were dropped from the study (both patients were discharged). Ultimately, 41 patients of the ICU group (pneumonia, n=18; acute exacerbation of COPD, n=14; ARDS, n=8; and acute exacerbation of asthma, n=1) and 42 patients of the general ward group (pneumonia, n=16; acute exacerbation of COPD, n=14; acute exacerbation of asthma, n=10, pneumoconiosis, n=1; and tuberculosis, n=1) were finally evaluated in this study. Figure 1 contains CONSORT diagrams on patient recruitment.
Clinical laboratory examinations
Blood sampling was conducted in all subjects in fasting state at 6:00 am. Serum and urine selenium levels were measured using the atomic absorption spectrometer-graphite furnace (VARIAN medical systems, Sydney, Australia). C-reactive protein (CRP) was measured using the Modular DPE (Hitachi Hitechnologies, Tokyo, Japan). Procalcitonin was measured using sandwich enzyme-linked immunosorbent assay (bioMérieux, Lyon, France).
In addition, to obtain APACHE II (acute physiology and chronic health evaluation II) scores, we evaluated the age, chronic health problems, rectal temperature within 24 hours of hospitalization, mean arterial pressure, heart rate, respiratory rate, arterial blood oxygen saturation, pH, Na+, K+, creatinine, hematocrit, WBC counts and Glassgow coma scale (11).
Outcome measures and assessment
During a one-week observational period, we monitored patient outcomes based on following measurements: (I) systemic inflammatory responses (CRP and procalcitonin); (II) prognosis of critically-ill patients (APACHE II scores); and (III) nutritional status (lymphocyte counts and albumin). Through the collection of serum and urine samples within 24 hours of hospitalization, we measured serum and urine selenium levels and systemic inflammatory indicators, such as CRP, procalcitonin and total WBC counts, and their fractions. Of the differential counts, lymphocyte counts were served as one of the indicators of nutritional status along with albumin levels (12-14). In accordance with a previously conducted study in patients staying at surgical ICU, it was confirmed that micronutrients including selenium reduced at the time of hospitalization were recovered to the normal range within 1 week (15). Therefore, in this study, to examine changes in measurements depending on treatment course after hospitalization, we evaluated all the indicators including serum and urine selenium levels at 4th and 7th day (procalcitonin at 7th day only) in the ICU group and at 7th day in the general ward group.
Subgroup analysis
Based on a mean cut-off value of serum selenium levels of 82 ng/mL (mean-standard deviation) obtained from healthy Korean adults (16), we also divided our clinical series of patients into two groups depending on serum selenium levels on admission: the lower serum selenium level group and the higher serum selenium level group. Thus, we assigned 36 and 47 patients to the lower serum selenium level group and the higher serum selenium level group. In the lower serum selenium level group, of the 36 patients, 7 were from the general ward group (19.4%) and 29 from the ICU group (80.6%). Then, we compared clinical characteristics, systemic inflammatory markers, prognostic and nutritional factors between the two groups.
Statistical analysis
Statistical analysis was done using the SPSS program version 13.0 (SPSS Inc., Chicago, IL, USA), for which categorical data was expressed using frequencies and percentages and quantitative data was expressed as mean ± standard deviation. We analyzed differences in mean serum selenium levels between the two groups using the t-test. In addition, we also compared changes in systemic inflammatory indicators and serum selenium levels depending on treatment course after hospitalization between the two groups using the t-test. For variables with a significant correlation with lower serum selenium levels, we performed a simple regression analysis. Moreover, to evaluate the effects of explanatory variables for serum selenium levels on admission, we also performed a logistic regression analysis. We also performed univariate and multivariate analyses. For variables showing a P value <0.05 on univariate analysis, we also performed a multivariate analysis. A P value of <0.05 was considered statistically significant.
Results
Baseline and clinical characteristics of the patients
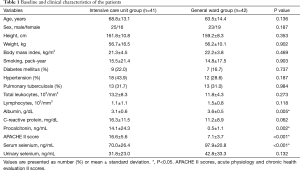
A total of 83 patients were divided into two groups: the ICU group (n=41) and the general ward group (n=42). Baseline and clinical characteristics of the patients, comorbidities, CRP, procalcitonin, APACHE II scores and serum and urine selenium levels are represented in Table 1. There were no significant differences in the age, sex, body mass index, the amount of smoking and comorbidities between the two groups. Serum selenium levels within 24 hours of hospitalization were significantly lower in the ICU group as compared with the general ward group (70.0±26.4 and 97.9±20.8 ng/mL, respectively, P<0.001). One of the systemic inflammatory markers, procalcitonin (14.1±24.3 and 0.5±1.1 ng/mL, respectively, P=0.002), and one of the prognostic factors, APCHE II scores (16.6±5.6 and 7.1±3.7, respectively, P<0.001) were significantly higher in the ICU group as compared with the general ward group. Moreover, of the nutritional markers, albumin was significantly lower in the ICU group as compared with the general ward group (3.1±0.6 and 3.6±0.5 g/dL, respectively, P=0.005).
Full table
CRP was higher in the ICU group as compared with the general ward group. But this did not reach statistical significance (P=0.062). In addition, there were also no significant differences in urine selenium levels between the two groups.
Changes in serum selenium levels and other markers depending on patient outcomes
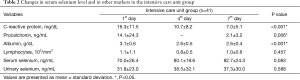
In the ICU group, there was a significant decrease in systemic inflammatory markers such as CRP and procalcitonin from baseline following the treatment process. In addition, there was a significant decrease in serum albumin levels, served as nutritional marker from baseline (Table 2).
Full table
In the general ward group, there was a significant decrease in serum CRP levels from baseline following the treatment process. In addition, there was an increase in serum selenium levels from baseline, as shown in the ICU group. But this did not reach statistical significance (Table 3). Urine selenium levels showed no significant difference in both groups.

Full table
Correlations of systemic inflammatory markers, prognostic and nutritional factors with baseline serum selenium levels
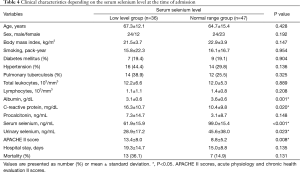
CRP and APACHE II scores were significantly higher and albumin was significantly lower in the lower serum selenium level group as compared with the normal serum selenium level group. In addition, length of hospital stay and the number of death cases were larger in the lower serum selenium level group as compared with the normal serum selenium level group. But the difference was not statistically significant (Table 4).
Full table
Interactional effects with explanatory variables for the outcome of baseline serum selenium levels
We performed a simple regression analysis of the inflammatory, prognostic and nutritional factors that may affect serum selenium levels on admission showing a significant increase or decrease in the lower serum selenium level group (Figure 2); these include lymphocyte counts, albumin, CRP and APACHE II scores. Lower serum selenium levels were associated with decreased levels of lymphocyte (R2=0.107, P=0.005) and albumin (R2=0.174, P<0.001). In addition, lower serum selenium levels had a correlation with an increase in baseline CRP (R2=0.059, P=0.041) and APACHE II scores (R2=0.209, P<0.001). But increased procalcitonin had no significant interactional effect on the decreased serum selenium levels.
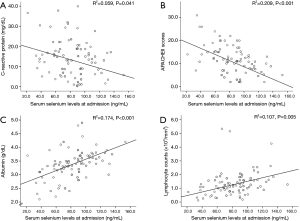
Factors associated with lower baseline selenium levels
As shown in Table 5, we performed both univariate and multivariate analyses to identify factors that are associated with lower baseline selenium levels. Thus, we found that higher CRP and APACHE II scores might be associated with lower baseline serum selenium levels. In addition, lower baseline albumin levels might also be associated with lower baseline selenium levels. Of these, lower baseline albumin levels and higher baseline APACHE II scores might have a significant correlation with lower baseline serum selenium levels on multivariate analysis.

Full table
Discussion
We found that baseline serum selenium levels were lower by 28% in the ICU group (70.0±26.4 ng/mL) as compared with the general ward group (97.9±20.8 ng/mL). Our results also showed that lower baseline serum selenium levels were associated with higher values of CRP and APACHE II scores and lower lymphocyte counts and serum albumin levels on admission. This indicates that lower serum selenium levels are associated with increased systemic inflammatory responses, poor prognosis and malnutrition in the early stage of hospitalization.
According to a previous study conducted in a French population, serum selenium levels were 68±23 ng/mL in medical and surgical patients and those who were admitted for multiple traumas. This showed that serum selenium levels were decreased as compared with normal healthy European controls (100±15 ng/mL) (7). In that study, of the 134 enrolled ICU patients, 78 (58%) were medical patients. Of these, ten and 21 patients were hospitalized for respiratory failure and infection, respectively. In this study, we enrolled only patients with respiratory diseases, with no history of surgery or trauma, who were admitted to a medical ICU or general wards. This result came from the analysis depending on the severity of the respiratory diseases and significantly lower values were confirmed in the critically ill patients. Considering the serum selenium level of healthy Koreans to be 112±30 ng/mL (16), the serum selenium levels (70±26 and 98±21 ng/mL) of these patients indicate that serum selenium levels can decrease when a patient is ill with respiratory diseases or when the disease is more severe. As described here, according to previous studies conducted in patients who were admitted to surgical ICU for major trauma, serum selenium levels were decreased since the admission and then maintained for a week and then gradually recovered thereafter. In addition, according to a previous study conducted in SIRS patients, serum selenium levels were decreased since the admission and then maintained for two weeks and then gradually recovered thereafter (7,15,17). Our results also showed that both the ICU group and the general ward group showed a decrease without a significant increase until at least one week after hospitalization since measurements were obtained. In the ICU patients, however, serum selenium levels were significantly lower as compared with normal values and then maintained for at least one week. This suggests that there is a significant correlation between significantly lower serum selenium levels in the early stage of hospitalization and a prognosis of patients.
In the current study, lower baseline serum selenium levels might have a correlation with systemic inflammatory responses based on higher serum CRP levels and a poor prognosis based on higher APACHE II scores. In particular, higher APACHE II scores, confirmed to be significant variables on a univariate analysis, were also confirmed to possibly have a significant correlation with lower serum selenium levels. These results are in agreement with previous reports that there was a significant negative correlation between APACHE II scores and serum selenium levels on admission in ICU patients with sepsis, trauma or burns (7). In this study, APACHE II scores were significantly higher in the ICU group as compared with the general ward group. Our results also showed that they were also significantly higher in the group where serum selenium levels were decreased on admission as compared with that with normal serum selenium levels. According to a study examining the mortality depending on APACHE II scores in 253 ICU patients, the predicted mortality was 11.0%, 35.5%, 70.3%, 91.0% and 92.0% when APACHE II scores were 3–10, 11–20, 21–30, 31–40 and ≥40 points, respectively (18). Similarly, the average APACHE II score of the lower serum selenium group was 13.4 with 36.1% of observed mortality and that of the normal serum selenium group was 8.8 with 14.9% of observed mortality in this study. As shown in our results, despite a lack of statistical significance, higher APACHE II scores had a correlation with a longer hospital stay and a higher mortality in the lower serum selenium levels group. The one week observation period was too short to confirm the difference in the mortality. This deserves further large-scale long-term follow-up studies.
The serum levels of CRP, served as an indicator of systemic inflammatory responses, were elevated in the lower serum selenium group. In consistent with this, a multivariate analysis also showed that higher serum CRP levels also had a significant correlation with low serum selenium. Of previous studies that had been conducted to examine the effects of selenium supplementation in adults, some reports showed that there was a significant negative correlation between serum selenium levels and serum CRP levels on admission (19). On the other hand, however, it has also been reported that there was no significant correlation between the two parameters (20). Moreover, procalcitonin is one of the reliable inflammatory markers, and it was significantly higher in the ICU group as compared with the general ward group. But it showed no significant difference between the lower serum selenium level group and the higher one. As described here, there are no consistent reports about correlations and interactions between inflammatory markers and serum selenium levels. Further studies are therefore warranted to examine whether systemic inflammatory responses have a significant correlation with lower serum selenium levels in critically ill patients.
Serum albumin and total lymphocyte counts, both of which were measured and then compared in the current study, are indicators that are used the most commonly for the screening of nutritional status (21-23). Serum albumin is an indicator of protein reserves (14,24). In addition, total lymphocyte counts are used as an indicator of immune defenses due to malnutrition (12,13). Thus, they are considered as a useful indicator of nutritional status. Of these two indicators, serum albumin levels were significantly lower in the ICU group as compared with the general ward group. They were also significantly lower in the lower serum selenium level group. Moreover, this was also confirmed on a multivariate analysis. According to a recent study showing that both indicators were useful for the nutritional screening and assessment, total lymphocyte counts of <1.6/µL were considered as decreased lymphocyte counts in patients with serum albumin levels of <3.5 g/dL (25). In this study, mean serum albumin levels were 3.1 g/dL in the ICU group with lower serum selenium levels and 3.6 g/dL in the general ward group with normal selenium levels. This leads to the speculation that there might be a difference in the nutritional status between the two groups.
As for parenteral nutrition such as selenium supplementation, the parenteral nutrition formula including selenium were not available in the hospital at the time of study implementation, the supplementation was not undertaken in both groups. However, there have been studies confirming whether selenium supplementation would be effective in improving the prognosis of critically ill patients with lower serum selenium levels. Thus, it has been reported that selenium supplementation was effective in reducing the occurrence of pneumonia, a length of hospital stay and the mortality in patients with burns or trauma (26-28). In recent years, however, according to the first systematic review in this series, selenium supplementation did not affect the mortality (29). Thereafter, three systematic reviews and meta-analysis showed that a parenteral supplementation of high-dose selenium would be effective in reducing the mortality in critically ill patients, particularly including those with sepsis (30-32). But these reviews are based on 9–12 randomized trials, each of which has been conducted under the different designs. Therefore, recent reviews have proposed that high-quality randomized trials be warranted (33). Moreover, there is also a paucity of baseline data about the difference in serum selenium levels in ICU patients between the different regional locations including Korea and other Asian countries with different soil and eating habits. Further studies are therefore warranted to examine the effects of selenium supplementation on the prognosis of ICU patients, for which our results are useful.
Selenium is mainly excreted through urine, and we measured urine selenium levels and thereby found that urine selenium levels were relatively lower in the ICU group despite a lack of statistical significance. Neither the ICU group nor the general ward group had significant changes in urine selenium levels until seven days since hospitalization. Following a comparison between the lower selenium level group and the higher one, however, urine selenium levels were significantly lower in the lower selenium level group. This is consistent with a previous study conducted in ICU patients with trauma showing that serum selenium levels were decreased since the date of hospitalization. But this study reported that the urinary excretion of selenium was left intact until 15 days of the onset of injury (15). Consistent with this, it has been reported that the urinary excretion of selenium did not change until 12 days but gradually decreased thereafter in young men taking non-selenium diet (33). In the current study, there was a significant decrease in urine selenium levels in the lower serum level group. According to previous studies conducted in patients with trauma or those for whom foods are restricted, there was a decrease in urinary selenium at 12 to 15 days after marked changes in serum selenium levels. This leads to the speculation that our clinical series of patients experienced a decrease in serum selenium levels two weeks prior to admission.
Because there is a paucity of selenium data in Korea whose soil or eating habits are quite different from those of western countries, our results are of significance in that the current study was conducted to examine the correlation between serum selenium levels, systemic inflammatory responses and prognostic indicators in ICU patients with respiratory diseases under the prospective design.
It is a limitation of the current study that we failed to clarify exact mechanisms by which serum selenium levels are decreased in ICU patients. In healthy individuals, selenium forms a complex with selenoprotein P (53%), glutathione peroxidase (39%) and albumin (9%) in vivo. This suggests that changes in the concentration of these molecules might be associated with lower serum selenium levels (34). Selenium is an essential trace element that is present at an amount of approximately 20 mg in the body. Selenium undertakes its role as a cofactor of glutathione peroxidases, which is a selenium-containing converts the reactive oxygen species into H2O depending on the disease conditions just like other enzymatic antioxidants such as superoxide dismutase (35). It is contained in selenoprotein and thereby plays a role in protecting cells from oxidation and suppressing the metabolism of inflammatory cells by lowering the degree of peroxidation of intracellular water content (5,34,36-38). Increased oxidative stress is associated with a poor prognosis of ICU patients. Thus, this explains the mechanisms by which lower serum selenium levels have an effect on the prognosis. Further prospective studies are warranted to overcome these limitations using a variety of variables. Moreover, we are confident that our results will provide a baseline data for such studies.
In conclusion, the serum selenium level at the time of admission was significantly decreased in the ICU group compared to the general ward group. In addition, there was a positive correlation of the serum selenium level with the albumin level and a negative correlation was identified between the serum selenium level and CRP and APACHE II score. This is possibly explained because low serum selenium levels are related to poor nutrition status at the time of admission and to systemic inflammatory responses and poor prognosis. This interaction should be considered when you interpret serum selenium levels in critically ill patients with respiratory diseases if you make a decision on selenium supplementation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of our medical institution (No. 2010-15) and written informed consent was obtained from all patients.
References
- Balk RA, Bone RC. The septic syndrome. Definition and clinical implications. Crit Care Clin 1989;5:1-8. [PubMed]
- Mishra V, Baines M, Wenstone R, et al. Markers of oxidative damage, antioxidant status and clinical outcome in critically ill patients. Ann Clin Biochem 2005;42:269-76. [Crossref] [PubMed]
- Shirai K, Yoshida S, Matsumaru N, et al. Effect of enteral diet enriched with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with sepsis-induced acute respiratory distress syndrome. J Intensive Care 2015;3:24. [Crossref] [PubMed]
- Heller AR, Rössler S, Litz RJ, et al. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit Care Med 2006;34:972-9. [Crossref] [PubMed]
- Forceville X. Seleno-enzymes and seleno-compounds: the two faces of selenium. Crit Care 2006;10:180. [Crossref] [PubMed]
- Hawker FH, Stewart PM, Snitch PJ. Effects of acute illness on selenium homeostasis. Crit Care Med 1990;18:442-6. [Crossref] [PubMed]
- Forceville X, Vitoux D, Gauzit R, et al. Selenium, systemic immune response syndrome, sepsis, and outcome in critically ill patients. Crit Care Med 1998;26:1536-44. [Crossref] [PubMed]
- Sakr Y, Reinhart K, Bloos F, et al. Time course and relationship between plasma selenium concentrations, systemic inflammatory response, sepsis, and multiorgan failure. Br J Anaesth 2007;98:775-84. [Crossref] [PubMed]
- Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr 2003;133:1463S-7S. [PubMed]
- Loscalzo J. Keshan disease, selenium deficiency, and the selenoproteome. N Engl J Med 2014;370:1756-60. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Shronts EP. Basic concepts of immunology and its application to clinical nutrition. Nutr Clin Pract 1993;8:177-83. [Crossref] [PubMed]
- Omran ML, Morley JE. Assessment of protein energy malnutrition in older persons, Part II: Laboratory evaluation. Nutrition 2000;16:131-40. [Crossref] [PubMed]
- Gibbs J, Cull W, Henderson W, et al. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg 1999;134:36-42. [Crossref] [PubMed]
- Berger MM, Cavadini C, Chiolero R, et al. Copper, selenium, and zinc status and balances after major trauma. J Trauma 1996;40:103-9. [Crossref] [PubMed]
- Kim YJ, Galindev O, Sei JH, et al. Serum selenium level in healthy Koreans. Biol Trace Elem Res 2009;131:103-9. [Crossref] [PubMed]
- Jang JY, Shim H, Lee SH, et al. Serum selenium and zinc levels in critically ill surgical patients. J Crit Care 2014;29:317.e5-8. [Crossref] [PubMed]
- Naved SA, Siddiqui S, Khan FH. APACHE-II score correlation with mortality and length of stay in an intensive care unit. J Coll Physicians Surg Pak 2011;21:4-8. [PubMed]
- Valenta J, Brodska H, Drabek T, et al. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med 2011;37:808-15. [Crossref] [PubMed]
- Mishra V, Baines M, Perry SE, et al. Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin Nutr 2007;26:41-50. [Crossref] [PubMed]
- Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med 1991;325:525-32. [Crossref] [PubMed]
- Elmore MF, Wagner DR, Knoll DM, et al. Developing an effective adult nutrition screening tool for a community hospital. J Am Diet Assoc 1994;94:1113-8, 1121; quiz 1119-20.
- Seltzer MH, Bastidas JA, Cooper DM, et al. Instant nutritional assessment. JPEN J Parenter Enteral Nutr 1979;3:157-9. [Crossref] [PubMed]
- Sullivan DH, Walls RC, Bopp MM. Protein-energy undernutrition and the risk of mortality within one year of hospital discharge: a follow-up study. J Am Geriatr Soc 1995;43:507-12. [Crossref] [PubMed]
- González Madroño A, Mancha A, Rodríguez FJ, et al. The use of biochemical and immunological parameters in nutritional screening and assessment. Nutr Hosp 2011;26:594-601. [PubMed]
- Berger MM, Spertini F, Shenkin A, et al. Trace element supplementation modulates pulmonary infection rates after major burns: a double-blind, placebo-controlled trial. Am J Clin Nutr 1998;68:365-71. [PubMed]
- Berger MM, Reymond MJ, Shenkin A, et al. Influence of selenium supplements on the post-traumatic alterations of the thyroid axis: a placebo-controlled trial. Intensive Care Med 2001;27:91-100. [Crossref] [PubMed]
- Collier BR, Giladi A, Dossett LA, et al. Impact of high-dose antioxidants on outcomes in acutely injured patients. JPEN J Parenter Enteral Nutr 2008;32:384-8. [Crossref] [PubMed]
- Avenell A, Noble DW, Barr J, et al. Selenium supplementation for critically ill adults. Cochrane Database Syst Rev 2004.CD003703. [PubMed]
- Heyland DK, Dhaliwal R, Suchner U, et al. Antioxidant nutrients: a systematic review of trace elements and vitamins in the critically ill patient. Intensive Care Med 2005;31:327-37. [Crossref] [PubMed]
- Huang TS, Shyu YC, Chen HY, et al. Effect of parenteral selenium supplementation in critically ill patients: a systematic review and meta-analysis. PLoS One 2013;8:e54431. [Crossref] [PubMed]
- Landucci F, Mancinelli P, De Gaudio AR, et al. Selenium supplementation in critically ill patients: a systematic review and meta-analysis. J Crit Care 2014;29:150-6. [Crossref] [PubMed]
- Levander OA, Sutherland B, Morris VC, et al. Selenium balance in young men during selenium depletion and repletion. Am J Clin Nutr 1981;34:2662-9. [PubMed]
- Forceville X, Mostert V, Pierantoni A, et al. Selenoprotein P, rather than glutathione peroxidase, as a potential marker of septic shock and related syndromes. Eur Surg Res 2009;43:338-47. [Crossref] [PubMed]
- Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta 2013;1830:3289-303.
- Manzanares W, Biestro A, Galusso F, et al. Serum selenium and glutathione peroxidase-3 activity: biomarkers of systemic inflammation in the critically ill? Intensive Care Med 2009;35:882-9. [Crossref] [PubMed]
- Brigelius-Flohé R, Banning A, Schnurr K. Selenium-dependent enzymes in endothelial cell function. Antioxid Redox Signal 2003;5:205-15. [Crossref] [PubMed]
- Costa NA, Gut AL, Pimentel JA, et al. Erythrocyte selenium concentration predicts intensive care unit and hospital mortality in patients with septic shock: a prospective observational study. Crit Care 2014;18:R92. [Crossref] [PubMed]