Risk factors for recurrence after sublobar resection in patients with small (2 cm or less) non-small cell lung cancer presenting as a solid-predominant tumor on chest computed tomography
Introduction
Lung cancer is a leading cause of cancer death globally (1). Lobectomy with mediastinal lymph node dissection has been the standard surgical procedure for stage I non-small cell lung cancer (NSCLC) (2,3). However, according to the National Comprehensive Cancer Network (NCCN) guideline for NSCLC (Version 4.2016), sublobar resection can be adopted for cases with poor pulmonary reserve; other major comorbidity that contraindicates lobectomy; or a peripheral nodule of ≤2 cm with at least one of the following: (I) pure adenocarcinoma in situ (AIS) histology; (II) nodule with ≥50% ground glass opacity (GGO) on chest computed tomography (CT); (III) radiologic surveillance confirms a long doubling time. Therefore, sublobar resection is usually performed for GGO-predominant tumors in our institution.
A GGO nodule on chest CT is usually considered a non-invasive or less invasive lepidic adenocarcinoma (4). Many studies have demonstrated that patients had a good prognosis after sublobar resection for GGO-predominant nodules (5-7). Accordingly, sublobar resection for GGO-predominant tumors is considered reasonable treatment. However, a good prognosis is not expected for patients who undergo a sublobar resection for lung cancer that presents as a solid-predominant nodule on chest CT because a radiologically solid tumor has more invasive pathologic characteristics than lung cancer with GGO features. Thus, sublobar resection is still controversial for solid-predominant lung cancer despite its small size (8). Until now, it has been difficult to establish guidelines for sublobar resection in radiologically solid-predominant lung cancer because of a lack of sufficient homogenous data (8). However, some studies reported that survival after sublobar resection for small-sized (≤2 cm) NSCLC is similar to that of lobectomy (9). Further, randomized trials including lung cancer with solid features are currently ongoing to validate these conclusions (CALGB 140503, JCOG 0802) (10,11).
The aim of this study was to evaluate outcomes for sublobar resection in small-sized (≤2 cm) NSCLC presenting as a solid-predominant tumor on chest CT. We compared the clinicopathologic characteristics and survival with those of GGO-predominant tumors. Moreover, we sought to identify risk factors related to the recurrence of small-sized NSCLC presenting as a solid-predominant nodule on chest CT to establish indications for sublobar resection.
Methods
Patients
From January 2004 to December 2014, 1,154 consecutive patients at Seoul St. Mary’s Hospital in Korea were diagnosed with NSCLC and underwent surgical resection. Of this population, 382 patients had tumors of ≤2 cm that were staged as clinical N0, and 121 patients underwent sublobar resection. Patients who underwent incomplete resection were excluded. No patient included in the study received preoperative chemotherapy or radiotherapy. Complete resection was defined as an absence of both macroscopic and microscopic residual cancer, especially in the resection margin. The study retrospectively enrolled 118 patients and assigned them to two groups according to their radiologic features: GGO-predominant tumor and solid-predominant tumor. Clinicopathological characteristics and survival were analyzed for both groups. Risk factors for recurrence were analyzed in the solid-predominant tumor group. Sublobar resection included a wedge resection and segmentectomy. Surgical indications for sublobar resection were a GGO-predominant tumor sized ≤2 cm or peripheral tumor accompanied by major comorbidities (cardiovascular or pulmonary disease) for which a lobectomy was contraindicated. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital at the Catholic University of Korea.
Radiological evaluation and preoperative staging
Clinical staging was determined by contrast-enhanced chest CT and F-18-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT scanning within 1 month before surgery. Primary lesions were evaluated using thin-section CT images. CT scans were obtained at full inspiration. GGO is characterized on CT scan by increased hazy opacities in the lung parenchyma with preservation of bronchial structures and vascular margins (12). Tumor diameter was defined as the largest axial diameter of the lesion on the lung window setting. Consolidation was defined as an area of increased opacification, which completely obscured underlying bronchial structures and vascular markings, and the diameter of the consolidation area was also measured on the axial image of the lung window setting. Tumors that had a consolidation diameter to tumor diameter ratio of <0.5 were defined as GGO-predominant tumors, whereas tumors with a ratio of ≥0.5 were defined as solid-predominant tumors. Each lung nodule was reviewed blindly on the preoperative CT scans by two thoracic surgeons.
Histological evaluation
All clinical specimens were examined by pathology specialists whose observations were recorded. Each tumor was reviewed for size, location, free resection margin, histologic types, differentiation, lymph node status, pleural invasion, and lymphatic invasion. The free resection margin was defined as the nearest length between the tumor and resection line. The margin/tumor ratio was also calculated using the free resection margin and maximum tumor diameter. These data were recorded on final histological examination reports. TNM staging was based on the seventh edition of the American Joint Committee on Cancer (AJCC) guidelines (13).
Statistical analysis
Clinicopathological factors were compared for each group. For the two groups, Student’s t-test or the Wilcoxon rank-sum test was used for continuous variables, and χ2 test or Fisher’s exact test was applied for categorical variables. Follow-up data for the interval between surgical resection and last follow-up visit were analyzed, and confirmed recurrence/death was used to calculate recurrence-free survival (RFS) using the Kaplan-Meier method. Survival for each group was compared using the log-rank test, and the Cox proportional hazards model for multivariate analysis was used to determine risk of recurrence in all patients and the solid-predominant group. All variables with a P of <0.1 in the univariate analysis were entered into a multivariate analysis. A backward stepwise regression procedure was used. A P value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS 19.0 software (IBM Corp, Armonk, NY, USA).
Results
A total of 118 patients were retrospectively enrolled in this study; of these, 45 patients had a solid-predominant tumor. Clinical characteristics of solid-predominant tumors were compared with GGO-predominant tumors (Table 1). In the solid-predominant tumor group, an older age and a higher mean maximum standardized uptake value (SUVmax) of FDG on PET scanning were characterized (69.1 vs. 60.3, respectively; P<0.001; 3.7 vs. 0.9, respectively; P<0.001). There were no statistical differences between the two groups with respect to sex, smoking status, involved lobes, forced expiratory volume in 1 second (FEV1), or serum carcinoembryonic antigen (CEA) level. All tumors were located peripherally. The mean percentage of diffusing capacity of carbon monoxide (DLCO) was lower in the solid-predominant tumor group than GGO-predominant tumor group (74.2% vs. 87.0%, respectively; P=0.001). The incidence of wedge resection in the solid-predominant tumor group was 73.3%, and it did not differ from that of the GGO-predominant tumor group. The incidence of postoperative complications was not statistically significant between the GGO-predominant tumor group (5.5%) and solid-predominant tumor group (15.6%) (P=0.101). There was no postoperative mortality in either group.

Full table
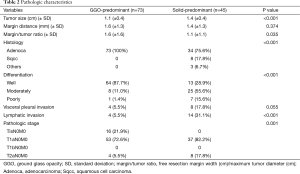
Pathologic characteristics were also analyzed in the solid-predominant tumor group and compared with those of the GGO-predominant tumor group (Table 2). Tumor size was larger in the solid-predominant group (1.4 vs. 1.1 cm, respectively; P<0.001). Mean margin distance and margin/tumor ratio in the solid-predominant tumor was 1.4 and 1.1 cm, respectively, and it did not differ from that of the GGO-predominant tumor group. The majority of solid-predominant tumors (75.6%) were adenocarcinomas. However, squamous cell carcinoma and other NSCLC types (two large cell carcinoma and one adenosquamous cell carcinoma) were also included in the solid-predominant tumor group. In the GGO-predominant tumor groups, all the tumors were adenocarcinomas. More moderately and poorly differentiated tumors were included in the solid-predominant tumor group (P<0.001). Patients with solid-predominant tumors experienced pleural invasion and lymphatic invasion more frequently than those with GGO-predominant tumors (P=0.055, P<0.001). Accordingly, T2aN0M0 was more frequently diagnosed in the solid-predominant tumor group.
Full table
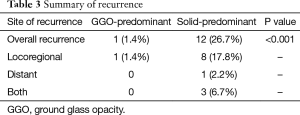
Median follow-up time for all patients was 884 days (range, 86–2,807 days), and recurrences were noted in 13 patients (Table 3). Only one locoregional recurrence occurred in a GGO-predominant tumor. However, 12 patients with solid-predominant tumors experienced recurrence. Five-year RFS in the solid-predominant tumor group and GGO-predominant tumor group was 64.9% and 95.5%, respectively (Figure 1).
Full table
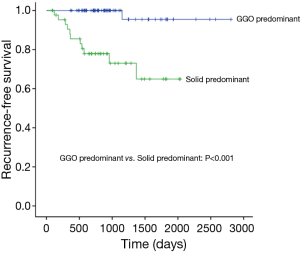
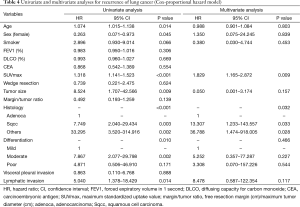
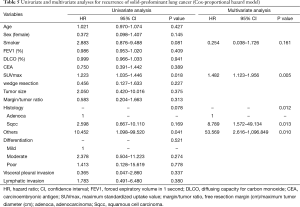
Table 4 shows our univariate and multivariate analyses using the Cox proportional hazards model to determine factors associated with recurrence after sublobar resection for NSCLC sized ≤2 cm. Multivariate analysis with age, sex, smoking status, SUVmax, tumor size, histologic types, tumor differentiation, and lymphatic invasion indicated that SUVmax [hazard ratio (HR) =1.829, 95% confidence interval (CI): 1.165–2.872; P=0.009] and histologic type (squamous cell carcinoma, HR =13.307, 95% CI: 1.233–143.557, P=0.033; other types, HR =36.788, 95% CI: 1.474–918.005, P=0.028) were significant risk factors for recurrence. Table 5 shows our univariate and multivariate analyses conducted to determine factors associated with recurrence after sublobar resection of tumors sized ≤2 cm in the solid-predominant tumor group. Multivariate analysis with smoking status, SUVmax, and histologic type indicated that SUVmax and histologic types were also significant risk factors for recurrence.
Full table
Full table
Discussion
Lobectomy has been a standard surgical technique for early-stage lung cancer. However, in some studies, patients who underwent sublobar resection demonstrated comparable outcomes with anatomical lobectomy for early-stage lung cancer (8,9). Sublobar resection has remained controversial, and two randomized controlled trials are ongoing (CALGB 140503, JCOG 0802). One recent study using a national cancer database in the United States demonstrated that patients who underwent sublobar resection for stage IA NSCLC had poorer oncologic outcomes (14). However, some studies reported that patients who had sublobar resection for stage IA NSCLC presenting as a GGO nodule had similar oncologic outcomes (6,7,15). Therefore, it might be possible that sublobar resection is suitable for selective cases. Although prospective studies are ongoing, sublobar resection may be a better treatment choice for small-sized GGO-predominant tumors because there are many advantages of sublobar resections, such as similar oncologic outcomes, preservation of pulmonary function, and low morbidity (16). For cases with a solid-predominant tumor, however, it is important to select appropriate indications for sublobar resection because oncologic outcomes with sublobar resection are not similar to those of lobectomy (17).
In the current study, the 5-year RFS was 95.5% with a GGO-predominant tumor and 64.9% with a solid-predominant tumor. This result was similar to other studies (6,9). For GGO-predominant tumors, most patients underwent an elective sublobar resection because of a strong probability for a good postoperative prognosis. However, sublobar resection was usually performed for high-risk patients (e.g., older or cardiopulmonary disease) with solid-predominant tumors. Therefore, comparisons of prognosis after sublobar resection were meaningless for patients with GGO-predominant and solid-predominant tumors. Instead, we wanted to determine the risk factors for recurrence after sublobar resection in all patients and the solid-predominant tumor group. As a result, the risk factors did not differ between all patients and the solid-predominant tumor group because most recurrences occurred in the solid-predominant tumor group. In this study, a high SUVmax and histologic types other than adenocarcinoma were significant risk factors for recurrence. Thus, for a solid-predominant tumor with a high SUVmax or non-adenocarcinoma type tumor, lobectomy may be a better treatment choice than sublobar resection.
Adenocarcinoma is heterogeneous, existing mostly in the form of mixed subtypes (18); therefore, its clinical course and prognosis can vary. Some reports also determined that the degree of lepidic pattern in a tumor was related to disease prognosis (19); a more favorable prognosis is seen with a lepidic pattern of ≥10% than a lepidic pattern of <10% (20,21). For the solid-predominant tumor group, adenocarcinoma could also be divided into a favorable prognosis tumor and poor prognosis tumor according to the degree of lepidic pattern. In the current study, many radiologically solid-predominant tumors with a lepidic pattern of ≥10% were included in the solid-predominant tumor group. As a result, patients with an adenocarcinoma type demonstrated a better prognosis than those with other histologic tumor types. If the solid-predominant tumor group could be subdivided according to lepidic pattern, it would provide us with more accurate results. However, the size of our study population was too small to perform it. A future study with large-scale data would be necessary to validate our results. Nevertheless, lobectomy should be considered in case of non-adenocarcinoma-like squamous cell carcinoma.
Resection margin distance is also an important factor for locoregional recurrence after sublobar resection (22). According to NCCN guidelines, segmentectomy and wedge resection should achieve parenchymal resection margin width greater or equal to the nodule size. Accordingly, we adopted a margin distance to maximum tumor size ratio (margin/tumor ratio). The mean margin/tumor ratio for the GGO-predominant group and solid-predominant group were 1.6 and 1.1, respectively. In other words, the mean resection margin was larger than the mean tumor size for both groups. Therefore, the margin/tumor ratio was not a significant risk factor for recurrence in our study.
In this study, we evaluated RFS instead of overall survival because with stage I disease, more patients succumbed to other causes than from cancer during the follow-up period (7). Further, RFS is a more accurate measurement of survival, as it reflects the biological behavior of the cancer rather than death secondary to unrelated factors. Besides, RFS is a more reliable measurement of cancer prognosis than overall survival because the solid-predominant tumor group included many high-risk patients.
This study had a number of limitations. First, this was a retrospective review conducted at a single center. Second, we obtained data from a single institution, and the number of cases was relatively small. Future multicenter studies with larger patient cohorts may remedy this problem. Third, a few histologic types were included in the current study. Most patients had adenocarcinoma and squamous cell carcinoma. Thus, it was difficult to identify the histologic type with the poorest prognosis after sublobar resection. However, except for adenocarcinoma and squamous cell carcinoma, the incidence of other histologic types was very low. Thus, our result could be helpful to determine risk factors for sublobar resection in small-sized NSCLC. Fourth, the accuracy of cN0 staging determinations may have benefited from invasive diagnostics in addition to imaging studies. However, invasive LN staging rarely yields positive results in instances of cN0 small-sized tumors found on chest CT and PET/CT scans, and given their high cost and related risks, they are generally performed only if nodal metastasis is suspected. Therefore, at our institution, surgical treatment was performed initially for patients diagnosed with cN0 small-sized tumors using chest CT and PET/CT scans.
In conclusion, small-sized solid-predominant NSCLC has more risk factors for recurrence and a poorer prognosis than GGO-predominant small-sized tumors after sublobar resection. The risk factors related to recurrence after sublobar resection in small-sized NSCLC are a high SUVmax and histologic types other than adenocarcinoma. The risk factors for small-sized solid-predominant tumors are also the same as those of small-sized NSCLC. Thus, a lobectomy should be considered for small-sized solid-predominant NSCLC in patients with a high SUVmax or non-adenocarcinoma types. Additional studies that include data from larger homogenous cohorts may validate these conclusions and provide more refined results.
Acknowledgements
The manuscript has been edited by native English-speaking experts at BioMed Proofreading, LLC.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital at the Catholic University of Korea.
References
- Torre LA, Siegel RL, Jemal A, et al. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Wilshire CL, Louie BE, Manning KA, et al. Radiologic Evaluation of Small Lepidic Adenocarcinomas to Guide Decision Making in Surgical Resection. Ann Thorac Surg 2015;100:979-88. [Crossref] [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [Crossref] [PubMed]
- Taioli E, Yip R, Olkin I, et al. Survival after Sublobar Resection for Early-Stage Lung Cancer: Methodological Obstacles in Comparing the Efficacy to Lobectomy. J Thorac Oncol 2016;11:400-6. [Crossref] [PubMed]
- Koike T, Kitahara A, Sato S, et al. Lobectomy Versus Segmentectomy in Radiologically Pure Solid Small-Sized Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1354-60. [Crossref] [PubMed]
- Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol 2010;5:1583-93. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. editors. AJCC cancer staging manual. 7th ed. New York: Springer, 2010.
- Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Sim HJ, Choi SH, Chae EJ, et al. Surgical management of pulmonary adenocarcinoma presenting as a pure ground-glass nodule. Eur J Cardiothorac Surg 2014;46:632-6; discussion 636. [Crossref] [PubMed]
- Okada M. Radical sublobar resection for small-diameter lung cancers. Thorac Surg Clin 2013;23:301-11. [Crossref] [PubMed]
- Hattori A, Suzuki K, Matsunaga T, et al. Is limited resection appropriate for radiologically "solid" tumors in small lung cancers? Ann Thorac Surg 2012;94:212-5. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Moon Y, Kim KS, Sung SW, et al. Correlation of histological components with tumor invasion in pulmonary adenocarcinoma. World J Surg Oncol 2014;12:388. [Crossref] [PubMed]
- Moon Y, Sung SW, Lee KY, et al. The importance of the lepidic component as a prognostic factor in stage I pulmonary adenocarcinoma. World J Surg Oncol 2016;14:37. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]


