Heart rate reduction may be a major determinant of vascular tone in esmolol-treated septic shock patients—although still remains to be confirmed!
Introduction
Over the last decade, the regulation of the autonomic nervous system by β1-adrenoreceptor antagonists in septic shock patients has been the subject of growing literature. In 2013, Morelli et al. published a randomized, controlled, open-label, single-center phase 2 trial investigating the efficacy of intravenous esmolol, a short acting β1-blocker titrated to lower heart rate (HR) in septic shock patients with severe tachycardia. This study found that esmolol was associated with a reduction in HR to the specified target range (80–94 bpm), maintained cardiac output, increased systemic vascular resistances along with a subsequent reduction in norepinephrine dose requirements (1). The mechanisms by which esmolol reduced norepinephrine requirement in this trial remain unclear. Indeed, although mean blood pressure (MBP) is the result of the product of cardiac output and systemic vascular resistance, vessels exhibit an extremely low density of β1-adrenoreceptors (2).
Study design and findings
In a recent study, Andrea Morelli et al. hypothesized that the esmolol-related reduction in HR would increase diastolic filling time and consequently stroke volume (SV) in septic shock. Such increase in SV could be associated with decreased arterial Elastance (Ea) and, thus, with improved vascular tone, thereby explaining the reduction in norepinephrine requirement to maintain adequate MBP. In this single center trial, adult patients with septic shock were included if they had persistent tachycardia >94 bpm at 24 hours of optimal resuscitation. Patients who required inotropic agents, or had significant valvular disease or arrhythmia, were excluded. Before and at 4 hours of intravenous esmolol titration to maintain HR in the range of 80 to 94 bpm, central and pulmonary hemodynamics, cardiac output, beat-to-beat estimation of SV (derived from arterial pressure waveforms), left ventricle ejection fraction by echocardiography, and Ea were recorded. All patients received identical resuscitation management (fluid, catecholamine, sedation) although inotropic drugs were not allowed during the study. Forty-five of the 116 screened patients were included, of which 73% were male. Patients had a mean SAPSII score of 54±17, a mean length of ICU stay of 18±17 days, and a 28-day survival rate of 49%. Compared to baseline, at 4-hours of esmolol infusion, Ea significantly decreased while SV increased and the dose of norepinephrine was reduced. Table 1 displays the main hemodynamic data reported in this study.

Full table
Commentary
Morelli et al. showed that in adults with septic shock and persistent tachycardia, intravenous treatment with esmolol was associated with a reduction in both HR and the need for norepinephrine. These authors suggested that the observed reduction in the dose of norepinephrine to maintain MBP >65 mmHg was a consequence of improved vasomotor tone, as indirectly assessed by changes in Ea.
This study has substantial limitations that prevent any definite conclusions regarding the mechanisms underlying the esmolol-associated reduced need for norepinephrine. First, it was designed as an uncontrolled observational study. Thus, only information on association and not on causality may be provided. At the very least, the authors should have designed alternate sequences of ‘on and off’ esmolol administration. The ultra-short half-life of esmolol would have permitted such design. Secondly, the generalizability of these findings is unclear. Indeed, almost 40% of these patients had persistent tachycardia after 24 hours of optimal resuscitation. Other recent trials, e.g., PROCESS and ARISE, reported mean HR values of 114 and 104 bpm at baseline, 97 and 94 bpm at 6 hours, and of 95 and 90 bpm at 24 hours. Accordingly, in the general septic shock population, there are much fewer patients with persistent tachycardia than that observed in the trials by Morelli et al. (3-6). Third, the indirect and imprecise assessment of vasomotor tone may not allow definite conclusions.
Septic shock is characterized by ventriculo-arterial uncoupling defined as systolo-diastolic biventricular dysfunction as well as vascular hyporesponsiveness to endogenous vasopressors (7). Ventriculo-arterial coupling (VAC) is described using the Pressure-Volume curves recorded from an intraventricular conductance catheter. Left ventricular inotropism can be investigated experimentally, by varying preload or afterload, drawing a series of Pressure Volume loops defining the End Systolic Pressure (ESP) Volume Relationship (Figure 1). The slope of this relationship, called End Elastance slope (Ees), is a reliable marker of load-independent cardiac function. ESP is a function of cardiac contractility, with SV and afterload roughly representing vasomotor tone in the aorta. Thus, Ea is defined by the slope of the relationship between SV and ESP, and can be estimated by the ESP/SV ratio. There is a linear relationship between systolic blood pressure and ESP, i.e., ESP =0.9 SAP (8). Hence, Ea can be estimated as equal to 0.9 SBP/SV (Figure 1). Ea is dependent on various factors including peripheral vascular resistances, arterial compliance, arterial impedance and systolic-diastolic interval times (9). VAC may be estimated by the Ea/Ees ratio (10). In healthy humans, the optimal value for Ea/Ees ratio is around 1, and a value of Ea/Ees ratio >1.36 reflects ventriculo-arterial uncoupling (9). As described above, ventriculo-arterial uncoupling may result from a decrease in Ea (e.g., vasoplegia in septic shock) and/or decrease in Ees (cardiogenic shock or septic cardiomyopathy). Morelli et al. assessed Ea using two relatively independent methods. They estimated SV from a single beat-to-beat analysis of the arterial catheter and from the thermodilution cardiac output. However, for Ea calculation, surprisingly, the authors used mean blood pressure as the numerator and not systolic blood pressure, which allows, as seen above, a better approximation of ESP. Mean blood pressure is far from reflecting ESP. Finally, these authors failed to appropriately assess VAC since they only estimated Ea and did not record the Ea/Ees ratio, although this was in fact performed in a previous study (11).
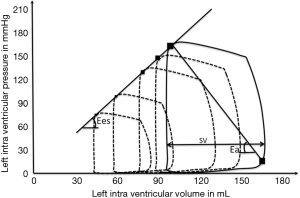
In addition, given the decrease in mean blood pressure, it is very difficult to link the reduction in norepinephrine requirement to esmolol effects on SV and Ea. Matching the MBP between the two time measurements would have strengthened the results on norepinephrine requirement. This methodological bias may also impact other hemodynamic parameters, particularly on the MBP-Pdic variable.
Morelli et al. further suggested that the anti-inflammatory effects of esmolol may also contribute to the observed changes in Ea. However, they failed to provide any supporting data, and one may argue that anti-inflammatory effects would unlikely have occurred this swiftly, i.e., after only four hours of treatment (12-15).
In summary, the study by Morelli et al. found that, in selected septic shock patients with persistent tachycardia, esmolol infusion may effectively control HR, improve stroke volume and Ea. Unfortunately, methodological biases in the assessment of vasomotor tone prevented any bona fide explanation on the mechanisms by which esmolol reduced the need for norepinephrine.
Acknowledgements
None.
Footnote
Provenance: This is an invited Commentary commissioned by the Section Editor Zhongheng Zhang (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua, China).
Conflicts of Interest: Antoine Kimmoun and Bruno Levy received a grant from Baxter for the Esmosepsis study (NCT02068287) and fees for consulting activity (Baxter and MSD). The other authors have no conflicts of interest to declare.
References
- Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 2013;310:1683-91. [Crossref] [PubMed]
- Karim F, Poucher SM. Beta-adrenoceptors in vascular capacitance responses to unloading of carotid baroreceptors in anesthetized dogs. Am J Physiol 1997;273:H1713-8. [PubMed]
- ProCESS Investigators, Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014;370:1683-93. [Crossref] [PubMed]
- ARISE Investigators; ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014;371:1496-506. [Crossref] [PubMed]
- Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015;372:1629-38. [Crossref] [PubMed]
- Quenot JP, Binquet C, Kara F, et al. The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Crit Care 2013;17:R65. [Crossref] [PubMed]
- Hatib F, Jansen JR, Pinsky MR. Peripheral vascular decoupling in porcine endotoxic shock. J Appl Physiol (1985) 2011;111: 853-60. [PubMed]
- Chemla D, Antony I, Lecarpentier Y, et al. Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol 2003;285:H614-20. [Crossref] [PubMed]
- Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985) 2008;105:1342-51. [PubMed]
- Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res 1985;56:586-95. [Crossref] [PubMed]
- Guarracino F, Ferro B, Morelli A, et al. Ventriculoarterial decoupling in human septic shock. Crit Care 2014;18:R80. [Crossref] [PubMed]
- Ackland GL, Yao ST, Rudiger A, et al. Cardioprotection, attenuated systemic inflammation, and survival benefit of beta1-adrenoceptor blockade in severe sepsis in rats. Crit Care Med 2010;38:388-94. [Crossref] [PubMed]
- Kimmoun A, Louis H, Al Kattani N, et al. β1-Adrenergic Inhibition Improves Cardiac and Vascular Function in Experimental Septic Shock. Crit Care Med 2015;43:e332-40. [Crossref] [PubMed]
- Mori K, Morisaki H, Yajima S, et al. Beta-1 blocker improves survival of septic rats through preservation of gut barrier function. Intensive Care Med 2011;37:1849-56. [Crossref] [PubMed]
- Suzuki T, Morisaki H, Serita R, et al. Infusion of the beta-adrenergic blocker esmolol attenuates myocardial dysfunction in septic rats. Crit Care Med 2005;33:2294-301. [Crossref] [PubMed]


