Use of laryngeal mask airway for non-endotracheal intubated anesthesia for patients with pectus excavatum undergoing thoracoscopic Nuss procedure
Introduction
Pectus excavatum (PE) is the most frequent congenital deformity of the chest wall in children and young adults. In 1998, the Nuss procedure was introduced as a minimally invasive alternative to the classic open Ravitch operation (1). For its obvious advantages of maintaining the normal function and flexibility of the chest wall, a “good” or “excellent” outcome in 98% patients, a relatively short operating time and minimal blood loss (2), the Nuss procedure has been accepted by more and more patients and surgeons.
Previously, general anesthesia using an endotracheal tube (ETT) with muscle relaxation was the standard for the Nuss procedure (1). However, adverse effects related to ETT, such as intubation-related trauma, increased risk of pneumonia, impaired cardiac performance, postoperative nausea and voice hoarseness, have prompted some surgeons to prefer the thoracoscopic procedure under non-endotracheal intubated anesthesia. The laryngeal mask airway (LMA) is a relatively new device that was introduced to clinical practice in the 1980s. Unlike ETT, the LMA is supraglottic and usually inserted blindly, thereby avoiding laryngoscopy. Therefore, it inevitably causes less intubation-related adverse events, such as vomiting, cough, hoarseness, sore throat, increased risk of pneumonia and impaired cardiac performance.
Considering these benefits, the choice of thoracoscopic Nuss procedure in combination with non-endotracheal intubated anesthesia could have the latent possibility of decreasing the invasiveness of the procedure and allowing quicker postoperative recovery. Therefore, we conducted the present study to determine the safety and feasibility of using the LMA for non-endotracheal intubated anesthesia for patients with PE undergoing the thoracoscopic Nuss procedure.
Methods
Patient recruitment
The study was designed as a pilot study and approved by the Human Ethics Committee of Guangdong General Hospital (Guangzhou, China). Written informed consent was obtained from the parents or legal guardians after they had been informed about the investigational nature of the study, the differences between LMA and ETT, the possibility that require conversion to an ETT may be necessary during the procedure, and the foreseeable outcomes. Eligibility criteria included a definite diagnosis of PE by the representative deformity of the chest wall, chest radiographic manifestations or computed tomography, and a willingness to be treated by thoracoscopic Nuss procedure. Exclusion criteria included an age of <10 years, preoperative hoarseness, increased risk of aspiration, mouth opening of <2.5 cm, a history of reactive airway or thoracic surgery, severe heart or respiratory tract diseases, gastroesophageal reflux, respiratory tract infections in the last 6 weeks, complications that were planned to be simultaneously managed by surgery and other surgical contraindications.
Anesthesia procedure
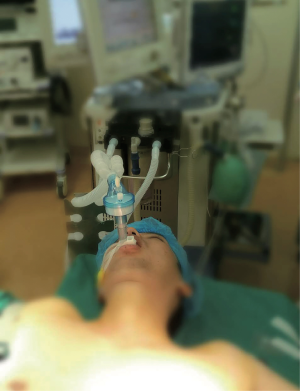
All patients underwent routine preoperative assessment, fasted for 8 hours before anesthesia and were anesthetized by two anesthetists with more than 5 years of experience. Standard monitoring included electrocardiogram, pulse oximetry, capnography and non-invasive blood pressure. Prior to the induction of anesthesia, an arterial line was established for blood gas analysis. For premedication, standard intravenous 0.25–1.0 mg of penehyclidine hydrochloride was applied based on weight. General anesthesia was induced and maintained with a target controlled infusion of 1.5–2.0 mg/kg propofol and a continuous infusion of 2–4 µg/kg/h fentanyl using a syringe pump. The LMA (Well Lead Medical Co., Ltd, Guangzhou, Guangdong, China), which was sized solely based on patient weight, was placed without any digital manipulation or other apparatus used. The cuff was inflated to the maximum volume and it was confirmed that there was no air leakage. After that, the airway device was attached to the anesthetic circuit (Fabius GS, Dräger, Lübeck, Germany) and anesthesia was maintained (Figure 1). Patients were ventilated with a pressure-controlled mode to obtain 7–10 mL/kg tidal volume, 12 per min respiration rate, and 1:2 inspiratory:expiratory rate. At the end of the procedure, the LMA was removed when the patient met standard extubation criteria (return of airway reflexes and regular spontaneous respirations).
Surgical procedure
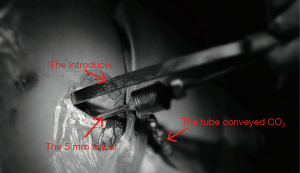
All surgical procedures were performed by the same team. The details of the procedure we used has been described previously (3). However, there is some emphasis that should be reiterated. First, after induction and anesthesia, patients were positioned supine to the right side on the table with both arms abducted approximately 70° from the chest wall. This position not only allowed good access to the lower chest wall and did not cause brachial plexus injury (2) but also ensured free movement of the thoracoscope (4); Second, the right end of the Nuss bar was shaped longer than the left for bar stabilization. Third, a 5-mm trocar was inserted through the same incision sites on the right side to maximize visualization (5). Fourth, to improve visualisation, CO2 was insufflated through the trocar and the pressure was maintained from 5 to 8 mmHg to keep the lungs out of the operative field (Figure 2). Fifth, when creating the retrosternal tunnel with a introducer, the thoracic entry and exit sites were placed close to the sternum to prevent disruption of the intercostal muscles (6). Sixth, after the retrosternal tunnel had been created, the introducer was pushed through the left incision and lifted in an anterior direction to pull the sternum and anterior chest wall out of their depressed position. Then, the malformed ribs were pressed forcefully several times to shape it. Next, after a 28-Fr transparent chest drain was fitted to the tip of the introducer and withdrawn from the right incision, the left end of the shaped Nuss bar was fitted to the right end of the drain hollow. Again the drain was withdrawn from the left incision and guided the Nuss bar across the critical retrosternal tunnel carefully under thoracoscopic guidance. The bar was rotated through 180° with the sternum being pushed upward and the PE was completely corrected. Seventh, one stabilizer was placed on the right side as close as possible to the thoracic entry sites to avoid rotation. And, the bar and the stabilizer were secured on the muscles with Polyester Sutures (7.0 metric, ETHICON, INC 2007, MB66.P33). Eighth, at the end of the procedure, a 12-Fr urethral catheter was placed through the right incision site (and the left incision site if left pneumothorax occurred) before the incisions were closed to evacuate the pneumothorax by expanding the lungs with positive pressure ventilation. Ninth, if two bars were needed, the surgical procedure for the second bar was approximately the same as above, with a few differences: the left end of the Nuss bar was longer than the right end, and the stabilizer was placed on the left side. Because it was difficult to place the two stabilizers on one side.
Conversion to open surgery or ETT
The surgeons made the decision to convert to open surgery if an uncontrolled bleeding happening, existing massive pleural adhesions or the outcome of the thoracoscopic Nuss procedure was not satisfying. For anesthesia, the indications for conversion to ETT included persistent hypoxemia (oxygen saturation on pulse oximetry of <90%), unstable hemodynamic status, or the decision to convert to open surgery.
Data collection and analyses
The following data were collected: time for LMA insertion, LMA insertion duration, operative length, blood loss, postoperative hospital stay, postoperative pain score, patients required conversion to ETT, and the peripheral O2 saturation (SpO2), end-tidal carbon dioxide (EtCO2) values, heart rate (HR), mean arterial blood pressure (MAP) at the time before induction and after LMA insertion, 5 min after lung collapse and expansion. Complaints of postoperative nausea, vomiting, cough, hoarseness, and sore throat were also recorded.
Results
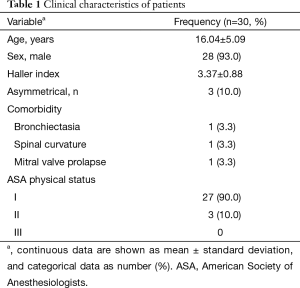
A total of 30 selected patients with PE were planned to undergo the thoracoscopic Nuss procedure using LMA for non-endotracheal intubated anesthesia in the Guangdong General Hospital between July 2015 and December 2015. The patients’ clinical characteristics are shown in Table 1. The mean age was 16.04±5.09 years; two patients were female. The Haller index was 3.37±0.88. At the time of the procedure, the American Society of Anesthesiologists (ASA) physical status was I in 27 patients and II in 3 patients.
Full table
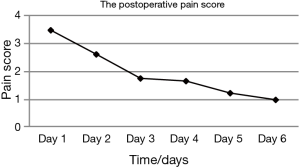
A total of 27 cases (90%) succeeded at the first attempt and two (6.7%) at the second, one patient (3.3%) required conversion to an ETT because of continuous air leak after the third attempt. The mean insertion time was 1.26±0.98 min, the mean LMA insertion duration was 123.27±55.645 min, operative length was 79.96±45.37 min, blood loss was 8.13±9.30 mL, and postoperative hospital stay was 4.13±1.30 days (Table 2). The SpO2, EtCO2, HR, and BP remained stable throughout all procedures (Table 3). Two patients experienced postoperative nausea and one complained of sore throat (Table 4). The mean postoperative pain score was 3.5±1.1 on the first day and gradually decreased (Figure 3). No patient required conversion to an open surgery; no gastro-esophageal reflux or in-hospital mortality occurred.
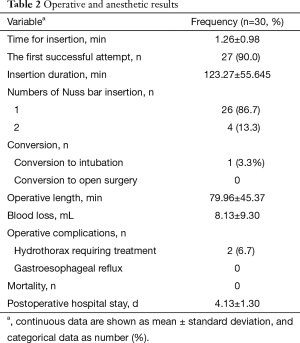
Full table
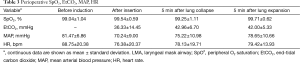
Full table
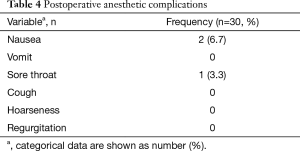
Full table
Discussion
PE presents as a depression of the sternum and costal cartilages, creating a funnel-shaped appearance of the chest and is the most common deformity of the chest wall. The incidence of PE is 0.12 per 100 persons (7). PE can not only cause physical problem, including dyspnea, decreased endurance, tachypnea with mild exertion, tachycardia, and chest pain (8), but also can affect psychological conditions, such as anxiety and depression (9). Moreover, Kelly et al. (7) confirmed that PE could impact survival. They found that patients with PE tended to die earlier, and those who survived past the age of 56 years tended to survive longer than their matched controls. It was reported that uncorrected PE persisted after childhood and often caused worsening symptoms with increasing age, and surgical repair can improve the body image, physical and psychosocial quality of life (8-11). The most established treatment option for PE is surgery. In 1998, the Nuss procedure (1) was introduced as a minimally invasive alternative to the classic open Ravitch operation. Since that time, as a minimally invasive procedure, it has been accepted by more patients and surgeons because of its advantages of avoiding extensive dissection, cartilage resection and osteotomy, which are integral components of the open Ravitch operation (12).
General anesthesia using an ETT with muscle relaxation was the standard for the Nuss procedure (1). However, as we know, ETT is associated with many intubation-related adverse events, such as vomiting, cough, hoarseness, sore throat, increasing risk of pneumonia and impaired cardiac performance. Furthermore, most patients with PE are juveniles. They have smaller tracheas and are more prone to such adverse events. To avoid endotracheal intubation and intubation-related adverse effects, thoracoscopic surgery without endotracheal intubation has recently been used for the management of numerous thoracic diseases, including pneumothorax (13), wedge resection of pulmonary tumors, segmentectomy, and lobectomy (14). Shao et al. (15) even reported a case of non-intubated complete thoracoscopic bronchial sleeve resection for central lung cancer. Typically, patients undergoing non-endotracheal intubation are anesthetized using regional anesthesia in a spontaneously single lung breathing status. But, this is impractical for the thoracoscopic Nuss procedure for severe pain and possible noncooperation of the juvenile patients. The use of the LMA, as one kind of non-endotracheal intubated technology, has been proven to result in an attenuated cardiovascular response compared with direct laryngoscopy and endotracheal intubation (16). The combination of thoracoscopic Nuss procedure in combination with non-endotracheal intubated anesthesia is a novel approach, and our experience in this study shows that it is clinically safe and technically feasible.
In the present study, 30 selected patients with PE were planned to undergo the thoracoscopic Nuss procedure using an LMA. The insertion success rate on the first attempt was 90% (27/30), even though most of them are juveniles whose tracheas are smaller. While in two cases the insertion was completed on the second attempt. Only one patient needed conversion to an ETT because of continuous air leakage. No major displacement of the LMA that needed conversion to an ETT occurred throughout the procedure in all cases, even at the moment of the malformed ribs were pressed forcefully and the lungs were expanded with positive pressure ventilation to evacuate the pneumothorax and the mean insertion duration exceeded 2 hours. The mean operative length was 79.96±45.37 min, which was similar to that we reported previously using an ETT (64.3±41.7 min) (3). These results suggest that the use of LMA do not influence the surgical procedure and it is as effective as ETT.
There are some concerns about using LMA for the thoracoscopic Nuss procedure. One major concern is its safety, especially when the CO2 was insufflated. Hemodynamic fluctuations of patients receiving ETT, which are difficult to avoid during or following direct laryngoscopy and tracheal intubation, may be attributed to the sudden contact of the tube tip with the bronchial wall during intubation (17). Conversely, after correct insertion, the tip of the LMA sits at the upper portion of the airway, which is characterized as non-endotracheal intubation and can be inserted without laryngoscopy that is necessary for edotracheal intubation. Therefore, the LMA may undoubtedly cause less perioperative hemodynamic fluctuations. The results of the present study demonstrated that the SpO2, EtCO2, HR, and MAP remained stable throughout the procedure in all cases (Table 3). Mizrak et al. (18), as well, reported that the LMA caused no change in HR or MAP values during or after airway placement in patients undergoing cystoscopy and thoracoscopic surgery. Furthermore, the SPO2 was maintained at 90% or higher and the EtCO2 remained less than 45 mmHg throughout the procedure in all cases, in spite of the CO2 was insufflated to improve visualization. These suggested that the use of LMA for non-endotracheal intubated anesthesia for selected patients with PE undergoing thoracoscopic Nuss procedure was clinically safe.
The other major concern regarding the use of LMA for the thoracoscopic Nuss procedure is its feasibility. As we know, most patients with PE are juveniles who have a smaller trachea and are more prone to intubation-related adverse events. The incidence of side effects (laryngeal pain, hoarseness and dysphonia) related to ETT in the early postoperative period has been reported to range from 15% to 50% (19). In such cases, a supraglottic airway device may be preferable. Unlike ETT, LMA is supraglottic and usually inserted blindly, avoiding laryngoscopy. Therefore, it inevitably causes less intubation-related adverse events, such as vomiting, cough, hoarseness, sore throat, increased risk of pneumonia and impaired cardiac performance. Hence, we hypothesized that the use of LMA for non-endotracheal anesthesia was particularly suitable for patients with PE undergoing the thoracoscopic Nuss procedure. The results of the present study showed that only two patients experienced nausea and one had a sore throat after surgery (Table 4). All of them recovered without any treatment. The mean postoperative pain score was 3.5±1.1 on the first day, which was acceptable, and declined gradually (Figure 3). There was no patient required conversion to an open surgery or died in-hospital; no cough, vomiting, hoarseness or regurgitation occurred. The mean postoperative hospital stay was 4.13±1.30 d, which was less than we reported previously (5.2±2.9 d) (3). It implied that this technology had a latent possibility of allowing quicker postoperative recovery. But, further investigation is needed to confirm. These results hinted that the use of LMA for non-endotracheal anesthesia for patients with PE undergoing the thoracoscopic Nuss procedure was technically feasible. Our hypothesis is further supported by many other studies. Peirovifar et al. (20) reported that postoperative cough, sore throat and difficulty in swallowing were significantly less in the LMA compared to the ETT group. Chun et al. (19) also reported that use of the LMA in general anesthesia for thyroid surgery had advantages over the ETT in decreasing patients’ subjective and objective voice symptoms, reducing the duration of symptoms, and relieving laryngopharyngeal symptoms.
We acknowledge that this study was limited by a small population and the lack of a control group who received ETT for comparison. Further investigation is encouraged to clarify the applicability and benefits of the use of LMA for non-endotracheal intubated anesthesia for patients with PE undergoing the thoracoscopic Nuss procedure.
Conclusions
In summary, our results showed that the use of an LMA in non-endotracheal intubated anesthesia for selected patients with PE undergoing a thoracoscopic Nuss procedure is clinically safe and technically feasible. However, larger prospective studies with appropriate control groups are needed to confirm our findings and to verify the latent possibility of this technology that could decrease the invasiveness and allow quicker postoperative recovery.
Acknowledgements
Funding: This study was funded by the Guangdong Province Medical Scientific Research Foundation (grant numbers A2015333, B2010004) and Guangdong Province Science and Technology Planning Project (grant numbers 20120318090, 2014A020212406) and Guangdong Natural Science Foundation (grant number 2014A030313636).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Human Ethics Committee of Guangdong General Hospital (Guangzhou, China) of No. 2015285H. Written informed consent was obtained from the parents or legal guardians after they had been informed about the investigational nature of the study, the differences between LMA and ETT, the possibility that require conversion to an ETT may be necessary during the procedure, and the foreseeable outcomes.
References
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [Crossref] [PubMed]
- Nuss D, Kelly RE Jr. The Minimally Invasive Repair of Pectus Excavatum. Oper Tech Thorac Cardiovasc Surg 2014;19:324-47. [Crossref]
- Zhang DK, Tang JM, Ben XS, et al. Surgical correction of 639 pectus excavatum cases via the Nuss procedure. J Thorac Dis 2015;7:1595-605. [PubMed]
- Pilegaard HK. Nuss technique in pectus excavatum: a mono-institutional experience. J Thorac Dis 2015;7:S172-6. [PubMed]
- Furukawa H, Sasaki S, William M, et al. Modification of thoracoscopy in pectus excavatum: insertion of both thoracoscope and introducer through a single incision to maximise visualisation. Scand J Plast Reconstr Surg Hand Surg 2007;41:189-92. [Crossref] [PubMed]
- Nuss D. Minimally invasive surgical repair of pectus excavatum. Semin Pediatr Surg 2008;17:209-17. [Crossref] [PubMed]
- Kelly RE Jr, Lawson ML, Paidas CN, et al. Pectus excavatum in a 112-year autopsy series: anatomic findings and the effect on survival. J Pediatr Surg 2005;40:1275-8. [Crossref] [PubMed]
- Jaroszewski DE, Fonkalsrud EW. Repair of pectus chest deformities in 320 adult patients: 21 year experience. Ann Thorac Surg 2007;84:429-33. [Crossref] [PubMed]
- Kelly RE Jr, Cash TF, Shamberger RC, et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics 2008;122:1218-22. [Crossref] [PubMed]
- Neviere R, Montaigne D, Benhamed L, et al. Cardiopulmonary response following surgical repair of pectus excavatum in adult patients. Eur J Cardiothorac Surg 2011;40:e77-82. [PubMed]
- Maagaard M, Tang M, Ringgaard S, et al. Normalized cardiopulmonary exercise function in patients with pectus excavatum three years after operation. Ann Thorac Surg 2013;96:272-8. [Crossref] [PubMed]
- Brochhausen C, Turial S, Müller FK, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg 2012;14:801-6. [Crossref] [PubMed]
- Rocco G, La Rocca A, Martucci N, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for spontaneous pneumothorax. J Thorac Cardiovasc Surg 2011;142:944-5. [Crossref] [PubMed]
- Hung WT, Hsu HH, Hung MH, et al. Nonintubated uniportal thoracoscopic surgery for resection of lung lesions. J Thorac Dis 2016;8:S242-50. [PubMed]
- Shao W, Phan K, Guo X, et al. Non-intubated complete thoracoscopic bronchial sleeve resection for central lung cancer. J Thorac Dis 2014;6:1485-8. [PubMed]
- Marietta DR, Lunn JK, Ruby EI, et al. Cardiovascular stability during carotid endarterectomy: endotracheal intubation versus laryngeal mask airway. J Clin Anesth 1998;10:54-7. [Crossref] [PubMed]
- Li Q, Li P, Xu J, et al. A novel combination of the Arndt endobronchial blocker and the laryngeal mask airway ProSeal™ provides one-lung ventilation for thoracic surgery. Exp Ther Med 2014;8:1628-32. [PubMed]
- Mizrak A, Kocamer B, Deniz H, et al. Cardiovasular changes after placement of a classic endotracheal tube, double-lumen tube, and Laryngeal Mask Airway. J Clin Anesth 2011;23:616-20. [Crossref] [PubMed]
- Chun BJ, Bae JS, Lee SH, et al. A prospective randomized controlled trial of the laryngeal mask airway versus the endotracheal intubation in the thyroid surgery: evaluation of postoperative voice, and laryngopharyngeal symptom. World J Surg 2015;39:1713-20. [Crossref] [PubMed]
- Peirovifar A, Eydi M, Mirinejhad MM, et al. Comparison of postoperative complication between Laryngeal Mask Airway and endotracheal tube during low-flow anesthesia with controlled ventilation. Pak J Med Sci 2013;29:601-5. [Crossref] [PubMed]