Perioperative outcomes and lymph node assessment after induction therapy in patients with clinical N1 or N2 non-small cell lung cancer
Introduction
Non-small cell lung cancer (NSCLC) represents a diverse group of tumor histologies, including squamous cell carcinoma, adenocarcinoma, and large-cell neuroendocrine carcinoma. Multiple trials have been conducted to determine the role and safety of induction chemotherapy (ICTx) with or without radiation in patients with resectable stage-2 or stage-3 NSCLC. While these trials tend to agree on the safety of induction therapy, surgery in addition to ICTx with or without radiation therapy (RT) in resectable lymph node (LN)-positive lung cancers has only relatively recently been shown to provide an overall survival benefit (1-5).
Early trials conducted over 20 years ago showed favorable short-term results with ICTx in stage-IIIA patients; however, the benefits were insignificant with long-term follow-up in the MD Anderson study and not reproducible in the Barcelona study (6-9). A 2015 meta-analysis of randomized controlled trials (RCTs) that included patients with cancers originally considered unresectable also showed no benefit to ICTx for stage-IIIA (N2) NSCLC in terms of overall survival (OS) (10). However, one of the included studies had a high mortality after pneumonectomy (26%), and further subset analysis demonstrated significantly higher OS with ICTx followed by lobectomy (11).
As previously noted, a meta-analysis of studies including only operative candidates demonstrated a 5% absolute increase in survival at 5 years across all stages of NSCLC with ICTx and surgery (1). Another study investigating the use of gemcitabine plus cisplatin prior to surgery found that ICTx had a significant benefit in treating stage-IIB/IIIA cancers with progression-free survival (PFS) at 3 years of 55.4% vs. 36.1% with surgery alone and an OS benefit of 23.4% at 3 years (12). When added to the previous meta-analysis, there is an OS benefit with ICTx for resectable late-stage NSCLC (HR =0.89) that is comparable to reported values for adjuvant CTx (HR =0.88) (4).
A potential benefit to ICTx over adjuvant CTx is the rate of compliance in terms of the number of planned cycles received, with less than 70% of patients beginning recommended adjuvant treatment and only two-thirds of those completing their protocol (3,13). Another potential benefit includes downstaging, which has been shown to independently improve OS (1,12,14-16). Furthermore, ICTx does not preclude use of adjuvant CTx or RT in the event of incomplete resection for patients able to tolerate further treatment.
With relatively new operative techniques being introduced in the surgical treatment of NSCLC, most notably the employment of robotic-assisted video-thoracoscopic (RAVTS) surgery, the present study seeks to elucidate the morbidity and early mortality associated with this approach in patients treated with induction therapy.
Methods
We conducted a retrospective analysis using prospectively collected data from all patients who underwent any thoracic surgical procedure at our institution by a single surgeon from September 2010 through May 2014. This study includes all patients who underwent RAVTS lobectomy, even those converted to open lobectomy. Our exclusion criteria selected out patients who had pathology other than NSCLC, including benign lesions or pulmonary metastasis. We excluded patients who required pneumonectomy, as these patients may have a different complication profile that would obscure analysis of complications attributable to ICTx. We also excluded those whose clinical nodal stage differed from clinical N1 (cN1) or clinical N2 (cN2). Patients were then divided into three groups: those who underwent ICTx, those who underwent ICTx plus radiation therapy (ICTx + RT), and those without induction therapy.
This study was conducted in accordance with the amended Declaration of Helsinki as outcomes research for quality assurance as part of our departmental Thoracic Oncology Clinical Research Database protocol. This database protocol was approved by our institution’s Scientific Review Committee (MCC#16512) and our university’s Institutional Review Board (IRB#Pro00002678), which considered this study as review of existing data and waived informed consent for this retrospective study. Nevertheless, all patients gave informed consent for our standard surgical procedure, which consisted of fiberoptic bronchoscopy, RAVTS lobectomy, or else RAVTS wedge resection followed by completion lobectomy, and then mediastinal lymph node dissection (MSLND), with possible thoracotomy. Some patients also gave informed consent for any anticipated en bloc chest wall and/or vertebral resection, with possible reconstruction. Through our institutional surgical informed consent, patients also gave permission to use surgery-related and tissue-related data for education and research purposes.
All of our patients undergo fiberoptic bronchoscopy by the operating surgeon after the induction of general anesthesia. After placement of the dual-lumen endotracheal tube, the patient is then placed in either the right or left lateral decubitus position, depending on which hemithorax the lesion is located. Our RAVTS lobectomy technique utilizes a three-port system, which includes a 4-cm camera port along the 6th intercostal space (ICS) at the anterior axillary line, which doubles as the assistant’s access port, and two 1-cm instrument ports along the 3rd ICS at the anterior axillary line and along the 9th ICS at the posterior axillary line. From September 2010 through December 2011, our group used the da Vinci® (Intuitive Surgical Corporation, Sunnyvale, CA, USA) “S”™ robotic surgical system, with the “Si”™ system being used from January 2012 to the present. Lobectomy is performed with the pulmonary vein divided first, then division of the pulmonary artery branch(es) and bronchus, and then completion of the pulmonary fissures. After delivery of the lobectomy within an endopouch through the 6th ICS incision, complete MSLND is performed. At the end of the procedure, a 32-French chest tube is introduced through the 9th ICS incision and connected to drainage at −20 cm H2O continuous suction.
Analyzed variables included patient demographics, operative time, intraoperative estimated blood loss (EBL), chest tube duration, hospital length of stay (LOS), and in-hospital mortality. Intraoperative complication rates were compared across all three groups, which included bleeding from a pulmonary artery or vein, recurrent laryngeal nerve (RLN) injury, and tracheal or bronchial injury.
Postoperative complication rates were also compared across all three groups, which included prolonged air leak lasting 7 days or longer, pneumonia, mucous plugs requiring intervention, pneumothorax after chest tube removal requiring reinsertion of chest tube, respiratory failure, aspiration, pulmonary embolism, hemothorax requiring intervention, and atrial fibrillation.
Clinical stage (cStage) was assessed through a systematic analysis of the patient’s history and physical, computerized tomography (CT) scan, positron-emission tomography (PET) scan, magnetic resonance imaging (MRI) studies, endobronchial ultrasound (EBUS), and/or cervical mediastinoscopy.
Pathologic stage (pStage) was determined by intraoperative findings and the final pathology report. Tumor histology and size, lymph node (LN) station number and location, and individual LNs were analyzed, and clinical and pathologic TNM staging were compared to determine rates of upstaging and downstaging.
All statistical analyses were performed using SPSS version 22.0. Mean, standard error of the mean (SEM), and range are reported for age, body mass index (BMI), tumor size, number of LN stations, and number of individual LNs. Intraoperative EBL, operative time, chest tube duration, and hospital LOS are expressed as median ± SEM, and range. Categorical data are expressed as count and percentage.
Results
A total of 256 patients underwent RAVTS pulmonary lobectomy between September 2010 and May 2014. Of these, 167 patients had a clinical LN stage other than cN1 or cN2, 4 patients underwent conversion to pneumonectomy due to hilar tumor involvement that precluded lobectomy, and 33 patients demonstrated non-NSCLC on final pathology, leaving 52 patients for evaluation. Of these patients, 7 underwent ICTx, 6 underwent ICTx + RT, and 39 went without induction therapy. Due to the relatively low sample size, some analyses were performed between those patients who underwent any form of induction therapy (13 patients) and those who did not receive induction therapy (39 patients).
There were no significant differences in patient demographics between the three groups (Table 1). The mean ages of each group were similar, with 61.1±4.3 years for patients having undergone ICTx, 66.3±2.1 years for patients having undergone ICTx + RT, and 68.4±1.7 years for patients without induction therapy. Patients who had undergone ICTx or ICTx + RT had slightly higher incidences of adenocarcinoma (71.4% and 66.7%, respectively) when compared to patients without induction therapy (57.1%).
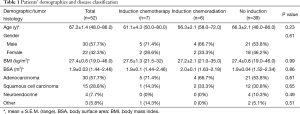
Full table
The pulmonary function status of the induction therapy and resection groups, reported in Table 2, was not significantly different. Patients undergoing induction had a mean forced expiratory volume in 1 second (FEV1) of 86.8%, while resection-only patients had a mean of 83.0% (P=0.07). Similarly, a mean diffusion capacity of the lung for carbon monoxide (DLCO) of 73% was noted in the induction group compared to 67.2% in the resection-only group (P=0.74).

Full table
Table 3 reports resection types, with patients having undergone ICTx + RT and patients without induction therapy more commonly having right lung pathology (100% and 69.2%, respectively, P=0.02) and a right upper lobectomy performed (83.3% and 41.0%, respectively, P=0.04). Patients having undergone ICTx more commonly had left lung pathology (71.4%, P=0.02) and a left upper lobectomy performed (57.1%, P=0.06). Overall intraoperative complication rate was 9.6%, with significant differences being the occurrence of a RLN injury (P=0.02) and the occurrence of a tracheal or bronchial injury (P=0.04). Overall postoperative complication rate was 44.2%, with the only significant difference being the occurrence of a pulmonary embolism (P=0.02) (Table 4).
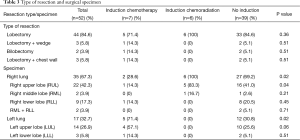
Full table
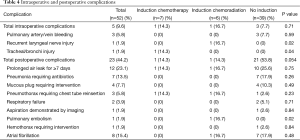
Full table
There was no significant difference in perioperative outcomes between the three groups (Table 5). Table 6 reports the perioperative outcomes between patients that received any form of induction therapy versus those who did not receive induction therapy, with no significant difference determined between groups. Patients having undergone any form of induction had higher rates of overall conversion (30.8%) compared to patients without induction therapy (12.8%), but this difference was non-significant (P=0.14).

Full table

Full table
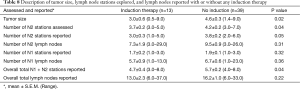
Table 7 reports tumor size and LN assessment for the three groups, with the only significant difference being tumor size (P=0.03). Table 8 reports the LN assessment between patients who received any form of induction therapy versus those who did not receive induction therapy, and a significant difference was found in the tumor size (P=0.02), the number of N2 stations assessed (P=0.04), the number of N2 stations reported (P=0.05), and the overall total LN stations reported (P=0.04).
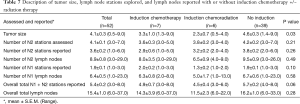
Full table
Full table
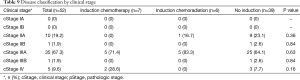
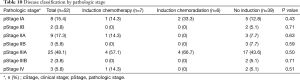
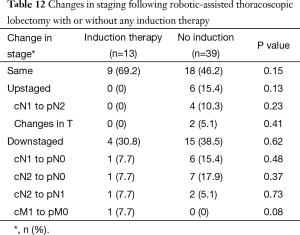
Tables 9,10 indicate cStage and pStage. There were no cStage-I patients versus 10 (19.2%) patients with pStage IA or IB, three of whom underwent induction treatment and seven of whom did not undergo induction therapy and thus were clinically overstaged as other than cStage I. Six (11.5%) patients were upstaged, none of whom received induction therapy, and 19 (36.5%) patients were downstaged (Table 11). Of the five patients clinically identified with distant metastasis, two underwent ICTx and three received no induction. After ICTx, one was downstaged to M0, compared to no revision of M status in those who did not receive induction therapy (P=0.04). Thirty-one percent of patients who underwent induction therapy were downstaged at operation, and 38.5% of patients who did not receive induction therapy had been clinically overstaged. There was no significant difference in overall upstaging or downstaging rates between the three groups or when comparing the upstaging or downstaging rates between patients who received any form of induction therapy versus those who did not receive induction therapy (Table 12).
Full table
Full table

Full table
Full table
Discussion
The rationale behind this study was to determine if induction therapy when used with RAVTS lobectomy would affect staging and perioperative or postoperative outcomes compared to RAVTS lobectomy alone in node-positive NSCLC. This study demonstrated that induction therapy, followed by RAVTS lobectomy, did not significantly affect staging or total number of intraoperative and postoperative complications.
Based on limited data in the ICTx and ICTx + RT groups by themselves, the data were first reviewed with respect to differences between the ICTx and ICTx + RT groups. Patient demographics, including age, gender, BMI, and pulmonary function, as well as tumor histology, were not significantly different between the two groups. Although patients undergoing ICTx + RT had significantly more right lung pathology and higher rates of right upper lobectomy (P=0.02 and P=0.04, respectively), while ICTx patients had significantly higher rates of left lung pathology (P=0.02), these differences were felt to be acceptable for combing the data from these groups for analysis as a single “induction therapy” group, as no other differences were found when analyzed separately.
This study demonstrated rates of downstaging with induction therapy (30.8%) slightly lower than those reported using video-assisted thoracoscopic (VATS) and open techniques (14,15,17). This difference in downstaging may have also been affected by incorrect clinical staging, which was observed at a minimum rate of 38.5% in our “no induction” group. Similar rates of downstaging were observed with and without induction therapy despite a significantly smaller tumor size at the time of surgery in patients undergoing induction therapy (P=0.02). There was also a trend toward more patients with stable disease in the induction therapy group and increased upstaging in the surgery alone group, but neither gained significance (P=0.15 and P=0.13, respectively). Pathologic downstaging at reported rates of 39–45% with thoracotomy and conventional VATS has been associated with improved 5-year survival in stage-IIIA NSCLC (3,18,19). Therefore, a lower rate of downstaging may represent a poorer prognosis for some patients with LN-positive NSCLC after RAVTS lobectomy. This lower rate of downstaging can be attributable to either less accurate preoperative diagnostic imaging or else better LN assessment, but more investigation is needed to determine if these findings are consistent with RAVTS among institutions.
Pathologic staging may have also been affected by N2 LN assessment, as significantly fewer N2 LNs were assessed and reported in those who underwent induction therapy than those who proceeded directly to surgery (P=0.04 and P=0.05, respectively). This may have led to over-reporting of stable and downstaged disease and limited the rate of upstaging in those who underwent induction therapy.
In analyzing the peri- and post-operative outcomes, the employment of induction therapy did not increase morbidity or early mortality significantly over RAVTS lobectomy alone. However, there was a significant difference with one occurrence each of RLN injury, tracheal/bronchial injury, and pulmonary embolism in those treated with induction therapy, as opposed to none in those with surgery alone (P=0.02, 0.04, and 0.02, respectively), with differences due to comparison of ICTx + RT and ICTx separately. By contrast, there was a trend toward more total post-operative complications with surgery alone, although it failed to reach significance (P=0.054). Evans et al. also reported a higher rate of RLN injury following induction therapy, but did not report on rates of pulmonary embolism or of tracheal/bronchial injury (18). Overall, complications were seen at a rate within reasonable range of those reported in recent literature with thoracotomy and conventional VATS (3,18,19).
In conclusion, either ICTx or ICTx + RT is a safe option in LN-positive NSCLC patients who are candidates for RAVTS lobectomy. This concurs with previous reports regarding morbidity and early mortality following post-induction thoracotomy and conventional VATS for NSCLC (3,18,19). These results lend support to the conclusions of multiple phase-III trials and meta-analyses that resection after induction therapy, particularly with lobectomy, can be safely performed so as to provide an OS benefit (1,3,4,11,12). Assessment of N2 LN stations was shown to be impaired after induction therapy in this study, however, which may have led to limitations in pathological staging. Such limitations could have undesired consequences, such as incorrect downstaging and subsequent erroneous communication of a better prognosis. Future research may focus on using PET-CT after RAVTS to assess for additional positive LNs in order to ascertain the relevance of this concern. Furthermore, the support of larger studies investigating induction therapy with RAVTS is needed to improve the power and validity of these results.
Study limitations
This study was not a RCT. Patients were selected for surgery and ICTx or ICTx + RT based on the experience and judgment of qualified surgical, medical, and radiation oncologists. While our surgeon operated solely at our institution, the medical and radiation oncologists were from multiple groups, introducing variability in the time course and scheduling of treatments. Patients were also able to decline recommended induction therapy without affecting their surgical candidacy. This option was not recorded. Thus, patient attrition and, therefore, selection bias are likely higher in the induction group, while potentially confounding the results of both groups. Lastly, as mentioned, RAVTS is a relatively new technique, and, as such, more investigation with larger patient enrollment is necessary to increase the power and validity of these results.
Acknowledgements
This manuscript is an update of our study previously given as an oral presentation at the Inaugural Asian Congress of Robotic Surgery, Hong Kong, December 16–17, 2015. This research was supported by 2014 Summer Scholarly Awards to Jessica Glover and to Emily Ng from the Scholarly Concentrations Program and by a 2015 Summer Scholarly Award to Kavian Toosi from the Office of Research, Innovation & Scholarly Endeavors (RISE), all at the University of South Florida (USF) Health Morsani College of Medicine.
Footnote
Conflicts of Interest: Eric M. Toloza and Jacques P. Fontaine have had financial relationships with Intuitive Surgical Corp. in the form of honoraria as robotic surgery proctors and observation sites. The other authors have no conflicts of interest to declare.
Ethical Statement: Data for this study was obtained through a Thoracic Oncology Program Clinical-Research Database protocol approved by the Moffitt Cancer Center Scientific Review Committee (MCC#16512) and the University of South Florida Institutional Review Board (IRB#Pro00002678), and written informed consent was obtained from all patients.
References
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [Crossref] [PubMed]
- Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol 2002;20:247-53. [Crossref] [PubMed]
- Pisters KM, Vallières E, Crowley JJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol 2010;28:1843-9. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer 1998;21:1-6. [Crossref] [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [Crossref] [PubMed]
- Felip E, Rosell R, Alberola V, et al. Preoperative high-dose cisplatin versus moderate-dose cisplatin combined with ifosfamide and mitomycin in stage IIIA (N2) non small-cell lung cancer: results of a randomized multicenter trial. Clin Lung Cancer 2000;1:287-93. [Crossref] [PubMed]
- Rosell R, Gómez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer 1999;26:7-14. [Crossref] [PubMed]
- Ren Z, Zhou S, Liu Z, et al. Randomized controlled trials of induction treatment and surgery versus combined chemotherapy and radiotherapy in stages IIIA-N2 NSCLC: a systematic review and meta-analysis. J Thorac Dis 2015;7:1414-22. [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [Crossref] [PubMed]
- Coleman BK, Curtis LH, Onaitis MW, et al. Adjuvant chemotherapy after resection of N1 non-small cell lung cancer: differential impact of new evidence on physician and patient decisions. J Thorac Dis 2015;7:243-51. [PubMed]
- Stefani A, Alifano M, Bobbio A, et al. Which patients should be operated on after induction chemotherapy for N2 non-small cell lung cancer? Analysis of a 7-year experience in 175 patients. J Thorac Cardiovasc Surg 2010;140:356-63. [Crossref] [PubMed]
- Yang H, Yao F, Zhao Y, et al. Clinical outcomes of surgery after induction treatment in patients with pathologically proven N2-positive stage III non-small cell lung cancer. J Thorac Dis 2015;7:1616-23. [PubMed]
- Toyooka S, Kiura K, Takemoto M, et al. Long-term outcome of induction chemoradiotherapy with docetaxel and cisplatin followed by surgery for non-small-cell lung cancer with mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg 2012;14:565-9. [Crossref] [PubMed]
- Yang CF, Adil SM, Anderson KL, et al. Impact of patient selection and treatment strategies on outcomes after lobectomy for biopsy-proven stage IIIA pN2 non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1607-13. [Crossref] [PubMed]
- Evans NR 3rd, Li S, Wright CD, et al. The impact of induction therapy on morbidity and operative mortality after resection of primary lung cancer. J Thorac Cardiovasc Surg 2010;139:991-6. [Crossref] [PubMed]
- Peer M, Stav D, Cyjon A, et al. Morbidity and mortality after major pulmonary resections in patients with locally advanced stage IIIA non-small cell lung carcinoma who underwent induction therapy. Heart Lung Circ 2015;24:69-76. [Crossref] [PubMed]


