Successful treatment of a bronchopleural fistula after en masse lobectomy
Introduction
We have advocated clamping and severing the pulmonary lobar root structure en masse for patients with inflammatory lung diseases (1). Among these methods, we have encountered concerns about en masse lobectomy. One of the complications to avoid is a bronchopleural fistula because the bronchial stump and vessels are too close to each other. We present successful treatment of a bronchopleural fistula after en masse lobectomy.
Case presentation
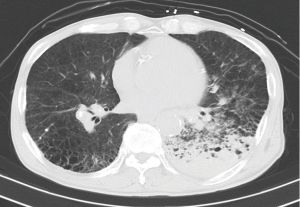
A 72-year-old man was brought to the emergency room because of massive hemoptysis. He had pulmonary hypertension, chronic obstructive pulmonary disease (COPD), and pneumoconiosis, and had been followed at our hospital. Computed tomography (CT) showed an infiltrative shadow with inflammatory changes in the left lower lobe (Figure 1). Therefore, we chose radiological intervention as the first option. After emergency transcatheter bronchial artery embolization of the left bronchial artery and seventh intercostal artery, the patient was hospitalized. The hemoptysis decreased, but continued, so we scheduled surgery on hospital day 18.
We planned a three-port thoracoscopic left lower lobectomy. The patient was placed in the full lateral decubitus position under general anesthesia with double-lumen intubation and slight flexion of the table at the mid-chest level. The three working ports were a 35 mm access port to extract the diseased lung, and two 12 mm ports for the flexible thoracoscope and various instruments. The inferior pulmonary vein was transected. Inspection revealed a largely fused fissure. We tried to separate the interlobar fissure, but exposing the lower lobe bronchus surface and pulmonary artery branch of the left lower lobe was difficult because of tight inflammatory adhesions. We chose en masse lobectomy as a last minimally invasive resort because the patient had cardiopulmonary problems, including COPD, pulmonary hypertension, and continued hemoptysis. We dissected the interlobar fissure as much as possible between the upper and lower lobes of the left lung and located the bifurcation of the lingular segmental branch of the pulmonary artery and the lingular bronchus. When the lower lobe was retracted cranially, we were able to identify the pulmonary artery trunk. Next, we confirmed the direction and thickness of the pulmonary parenchyma and the lower lobe bronchus/artery. Before stapling, we applied compression to exude fluid from the pulmonary parenchyma, which helped to ensure more consistent thickness of the targeted tissue (pre-compression). We used a 45-mm-long stapler (Echelon Flex ENDOPATH Stapler; Ethicon Endo-Surgery, Cincinnati, OH, USA), loaded with green-cartridge staples. The operating time was 420 min. The blood loss was 600 mL, and no blood transfusion was needed. Pathological examination of the resected specimen showed no malignancy, pneumoconiosis, or remarkable emphysematous change. The chest tube was removed on postoperative day 2.
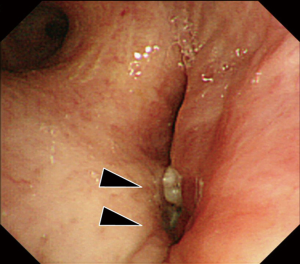
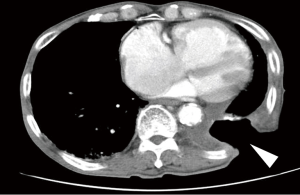
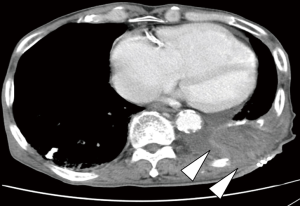
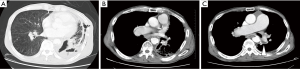
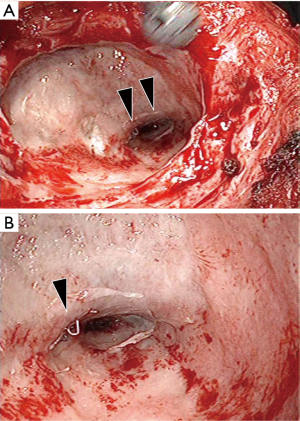
The patient complained of general fatigue and fever on postoperative day 14. Chest CT showed a new pleural effusion in the left pleural cavity and a contrast material defect at the pulmonary artery stump (Figure 2). In addition, bronchoscopy demonstrated dehiscence of the left lower bronchial stump (Figure 3). We diagnosed a bronchopleural fistula after en masse lobectomy. Therefore, the Clagett window procedure was scheduled on postoperative day 16 and parts of the ninth and tenth ribs were resected. The bronchopleural fistula was located at the edge of the bronchial stump of the left lower lobe. The pulmonary artery stump, which abutted the bronchial stump, was covered completely with connective tissue, and there was nothing to cause bleeding (Figure 4). The operating time was 133 min and there was little blood loss (Figure 5). We continued to exchange gauze after the second operation until the pleural space was clean.
The Clagett window was closed 3 months after the initial operation using a latissimus dorsi muscle flap. The operating time was 185 min and the blood loss was 182 mL (Figure 6). The chest drain was removed on postoperative day 2, and the subcutaneous drain was removed on postoperative day 7. He was discharged 18 days after the last operation.
He was healthy after discharge from the hospital, but died of a heart attack 33 months after the initial operation. He had no hemoptysis, and a CT-directed autopsy showed no pleural effusion, pneumonia, or remarkable changes.
Discussion
The bronchopleural fistula was difficult to avoid because the bronchial stump and vessel were too close to each other. We were concerned that the bronchopleural fistula would cause a bronchovascular fistula, but none developed in this case. The possibility of a bronchovascular fistula after en masse lobectomy should be considered more often (2). We have never experienced a potentially fatal complication after en masse lobectomy. Lewis et al. (3) also reported no bronchopleural or bronchovascular fistulas in 400 cases undergoing simultaneous stapling lobectomy, which is very similar to en masse lobectomy.
A bronchovascular fistula is caused by airway dehiscence, infection, or erosion of the pulmonary artery or a vascular suture line. A bronchial stump with tissue separating the bronchial and vascular suture lines has been reported to help prevent this complication (4). Actually, in this case, we found the bronchopleural fistula at the edge of the bronchial stump of the left lower lobe. The pulmonary artery stump, which abutted the bronchial stump, was completely covered with connective tissue, and there was nothing to cause bleeding. We carefully staple the pulmonary root to surrounding tissues in cases of benign disease. Surrounding tissues with dense adhesions were present between the bronchi and pulmonary vessels. Thus, the space between the bronchus and vessel was filled with tissue, such as pulmonary parenchyma or lymph nodes, and the other tissues covered the fistula. Second, the CT scan of the bronchopleural fistula showed a contrast material defect in the pulmonary artery stump, indicating that the inside of the pulmonary artery stump was lined with a thrombosis.
In a recent experimental swine model of en masse lobectomy (5), the pulmonary artery stump healing consisted of intraluminal reformation of the intima and media, and the media tissue fused and the pulmonary artery stump closed completely, histologically. In comparison, the bronchial stump healing consisted only of extraluminal coverage with fibrotic tissue. That is, the bronchial stump was attached only by the staples, and covered with fibrotic tissues, with no histological fusion.
In summary, we consider en masse lobectomy as an alternative, necessary technique in critical or unexpected situations, despite this case of bronchopleural fistula, which was managed successfully.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Kamiyoshihara M, Igai H, Ibe T, et al. Pulmonary lobar root clamping and stapling technique: return of the "en masse lobectomy". Gen Thorac Cardiovasc Surg 2013;61:280-91. [Crossref] [PubMed]
- Yim AP, Ho JK. Malfunctioning of vascular staple cutter during thoracoscopic lobectomy. J Thorac Cardiovasc Surg 1995;109:1252. [Crossref] [PubMed]
- Lewis RJ, Caccavale RJ, Bocage JP, et al. Video-assisted thoracic surgical non-rib spreading simultaneously stapled lobectomy: a more patient-friendly oncologic resection. Chest 1999;116:1119-24. [Crossref] [PubMed]
- Kawahara K, Akamine S, Takahashi T, et al. Management of anastomotic complications after sleeve lobectomy for lung cancer. Ann Thorac Surg 1994;57:1529-32; discussion 1532-3. [Crossref] [PubMed]
- Murakami J, Ueda K, Hayashi M, et al. Simultaneous stapling of the lobar bronchus and pulmonary artery: is it actually dangerous? Interact Cardiovasc Thorac Surg 2016;22:671-3. [Crossref] [PubMed]