Impact of pre-transplant pulmonary infection developed in horizontal laminar flow unit on the outcome of subsequent allogeneic hematopoietic stem cell transplantation
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an effective and curative therapy for hematological malignancies (1); however, the outcome of treatment is closely associated with the patient’s condition during transplantation. Before the procedure, the recipients need to receive multiple courses of chemotherapy, myeloablative conditioning, and prophylactic immunosuppressive therapy against graft versus host disease (GVHD), resulting in an impaired immune function and agranulocytosis. Prospective studies have reported that the incidence rate of early-stage infections before the transplant procedure is 54% (2), among which, pulmonary infection is a common problem (3). For patients with hematological malignancies who receive chemotherapy to achieve and maintain a complete response, allo-HSCT needs to be performed early due to the risk of recurrence and infections. Unfortunately, the limited bed number in horizontal laminar flow unit (HLFU) at many hospitals means that patients have to wait for a certain length of time before transplantation, and during which they may develop a pulmonary infection. Patients with an infection would still be eligible for allo-HSCT after completing antibiotic combination therapy, though (4); yet no clear conclusion has been drawn on the allo-HSCT outcome in patients with pre-transplant pulmonary infection. Therefore, our study aimed to evaluate the allo-HSCT outcome in those who had developed a pulmonary infection in HLFU and received antibiotic therapy and symptomatic treatment before transplantation.
Methods
Ethics statement
This study was approved by the Ethics Committee of Peking University People’s Hospital (No. [2012]26), and it was conducted in accordance with the Declaration of Helsinki. All subjects gave written informed consent before participating in the study.
Subjects
From January 1, 2012 to December 31, 2012, consecutive patients who received allo-HSCT without in vitro T-cell depletion at Peking University Institute of Hematology were included in this study if they met the following criteria: (I) those with hematological malignancies (e.g., leukemia, myelodysplastic syndromes, and lymphoma); and (II) those who underwent related donor hematopoietic stem cell transplantation (HSCT).
Transplant procedure and GVHD prophylaxis
The transplant procedure and modified donor lymphocyte infusion were consistent with those performed in previous studies (5,6). The transplant procedure, including the conditioning regimen, GVHD prophylaxis, and stem cell collection and supportive care, has been described in our previous reports (6,7). The agents used in the conditioning regimens were: cytosine arabinoside (Ara-C, 4 g/m2/d for 2 days), busulfan [Bu, 0.8 mg/kg intravenously (i.v.) every 6 h for 12 doses], cyclophosphamide (Cy, 1.8 g/m2/d for 2 days), simustine (Me-CCNU, 250 mg/m2/d for a single dose), anti-human thymocyte immunoglobulin (ATG, 2.5 mg/kg/d i.v. for 4 days), and total body irradiation (TBI, 770 cGy for a single dose). In this study, some patients received a Bu-based conditioning regimen that consisted of Ara-c (days 10 and 9), Bu (days 8, 7, and 6), Cy (days 5 and 4), ATG (days 5 to 2), and Me-CCNU (day 3), whereas the other patients were treated with a TBI-based conditioning regimen that included TBI (day 6), Cy (days 5 and 4), ATG (days 5 to 2), and Me-CCNU (day 3). All transplant recipients received immunosuppressive treatment with cyclosporine A, mycophenolate mofetil, and short-term methotrexate for prophylaxis against acute GVHD. For the transplantation, granulocyte colony-stimulating factor-mobilized bone marrow cells plus peripheral blood stem cells for graft infusions was used.
Infection prophylaxis and monitoring measures
A pulmonary function test and high-resolution computed tomography (HRCT) were performed as routine examinations; patients were assigned to the HLFU in reverse isolation. Chest radiography examinations were performed weekly from conditioning (day 10) until neutrophil engraftment. Berberine and norfloxacin were given orally for intestinal sterilization until engraftment. Intravenous, prophylactic, broad-spectrum antibiotics that provided coverage against Pseudomonas aeruginosa were used to prevent infection due to agranulocytosis. All patients received oral fluconazole (0.2 mg/day) for the primary prevention of candidiasis from the first day of conditioning to day 70, and intravenous itraconazole (200 mg, every 12 h, day 1; 200 mg/d afterward) was administered as secondary prevention against an invasive fungal infection. The oral administration of compound sulfamethoxazole (1 g, twice per day) was initiated to prevent pneumocystis infection; the dosing frequency was decreased to twice per week after day 30, and it was discontinued 3–6 months after transplantation. Intravenous ganciclovir (5 mg/kg, every 12 h, days 9 to 2) was used to reduce the risk of cytomegalovirus (CMV) infection. Oral aciclovir (400 mg, twice per day) was administered to prevent herpes simplex and varicella-zoster virus from day 1 to 12–18 months after transplantation. Tests for CMV DNA, Epstein-Barr virus (EBV), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level as well as 1,3-β-D-glucan assay (G assay) and galactomannan test (GM test) were performed once to twice per week. A throat swab culture was performed weekly until neutrophil engraftment. Once the patient’s symptoms were suggestive of pulmonary infection, pathogen screening was started immediately to identify bacteria, fungi, Pneumocystis jirovecii, antibodies against atypical pathogens, CMV, EBV, adenovirus, and influenza A virus. To do this, specimens were obtained from the sputum, blood, and bronchoalveolar lavage fluid if necessary; moreover, arterial blood gas analysis and chest HRCT were performed when a pulmonary infection was highly suspected (8).
Identification of pulmonary infections
On the basis of the diagnostic criteria for pulmonary infections (9,10), a clinical diagnosis was established if the following conditions were met: (I) the presence of at least one symptoms of cough, sputum, chest tightness and short of breath; (II) moist crackles throughout the lung fields; (III) a fever; and (IV) radiographically confirmed inflammatory infiltration of the lung tissue.
Evaluation of immune reconstitution
Data for the white blood cell (WBC) count, absolute lymphocyte count, and absolute monocyte count were obtained directly from patients’ routine blood tests on day 30 after HSCT.
Fresh peripheral blood samples were labeled with the monoclonal antibody for flow cytometry to analyze the following surface antigens: CD19, CD3, CD4, CD8, CD25, CD28, CD45RA, and CD45RO. The absolute number of peripheral blood cells was calculated to determine the percentages of CD19+ cells, CD3+ T cells, CD3+CD4+ T cells, CD3+CD8+ T cells, CD3+CD4−CD8− T cells, CD3+CD8+CD28− T cells, CD3+CD8+CD28+T cells, CD3+CD4+CD28− T cells, CD3+CD4+CD28+ T cells, CD4+CD45RO+CD45RA− T cells, CD4+CD45RO+CD45RA+ T cells, CD4+CD45RA+CD25+ T cells, and CD4+CD45RO+CD25+ T cells. Data acquisition and analysis were performed with Cell-Quest software (Becton-Dickinson, San Jose, CA). There were 24 indicators for the T-cell subgroups.
Statistical analysis
The overall survival (OS) was calculated in months from the first day after allo-HSCT to December 2012 or death; the OS was estimated with the Kaplan-Meier method. Log rank and Breslow tests were used to analyze differences between groups. Statistical Package for Social Sciences software, version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for data analysis. Two-sided P values <0.05 were considered significant.
Results
Patient characteristics
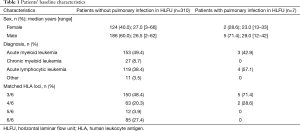
From January 2012 to December 2012, 332 patients with hematological malignancies received allo-HSCT at Peking University Institute of Hematology. We excluded 15 cases of unrelated donor allo-HSCT; therefore, 317 cases with related donors were included in our study (Table 1).
Full table
Among 317 patients who underwent allo-HSCT from related donors, 7 had chest tightness, shortness of breath, a cough, a body temperature above 38 °C, and other symptoms in HFLU before transplantation was performed. The findings on bedside chest radiography showed an incidence rate of 2.21% for pulmonary infection.
All patients who developed a pre-transplant pulmonary infection in HLFU (n=7) received a human leukocyte antigen (HLA)-haploidentical allo-HSCT (Table 2); of those, 2 died early after transplantation due to complications. The donor of one death case was a woman, and the donors of 5 patients who survived longer than 3 years were all men.

Full table
All patients in the group with a pre-transplant pulmonary infection who survived longer than 3 years suffered from a recurrent pulmonary infection after allo-HSCT. The incidence of post-transplant pulmonary infection was higher in the group with a pre-transplant pulmonary infection than in the group without [100% (5/5 patients) vs. 38.1% (118/310), χ2=5.542, P=0.019]. No statistical difference was found in terms of the survival rate between the two groups. Until December 2012, patients’ primary diseases were in complete remission.
Survival analysis
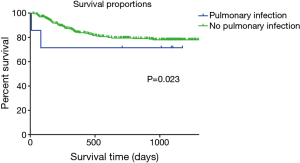
The Breslow test showed a lower survival rate in patients with a pre-transplant pulmonary infection in the HLFU than in patients without a pulmonary infection (n=310, OS: 28.4 vs. 42.4 months; Breslow test: P=0.023; log rank test: P=0.083) (Figure 1). Findings of the Breslow test rejected the null hypothesis (H0), whereas results of the log-rank test did not; therefore, the short-term difference in the survival rate between the two groups was considered more significant than that in the long-term.
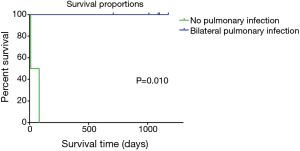
The survival rate was lower in patients who had a pre-transplant pulmonary infection accompanied with bilateral pleural effusion than in those without (n=5, OS: 1.5 vs. 36.3 months; Breslow test: P=0.010; log rank test: P=0.008; Figure 2). Results of the log rank test and Breslow test consistently led to an observation that short-term and long-term differences were both significant.
Wilcoxon rank sum test
During the first month after transplantation, patients with a pre-transplant pulmonary infection showed higher level of CD4CD45RO+CD45RA− [median =96.57 [88.11–98.76] vs. median 88.95 (6.67–97.66); P=0.008] and lower level of CD4CD45RO−CD45RA+ cells [median =0.825 (0.32–2.15) vs. median =3.51 (0.09–75.00); P=0.033] compared to those without. There were no statistically significant differences in terms of the other 22 indicators for the T-cell subgroups.
In the second month after transplantation, the group with a pre-transplant pulmonary infection had a lower WBC count than the group without a pre-transplant pulmonary infection [median =2.31 (1.83–4.69) vs. median =4.17 (0.78–25.69), P=0.017]. No statistically significant difference was found for the other 23 indicators for the T-cell subgroups.
In the third and fourth months after transplantation, we found no differences in all the 24 indicators for the T-cell subgroups between the two groups.
Findings of clinical investigations for pulmonary infection
Seven cases of pre-transplant pulmonary infection developed in HLFU were identified. In these patients, the results of CMV DNA, EBV, G assay, and GM test were within normal range. The oropharyngeal swab, sputum, and blood culture were negative. The CRP level [median =114 mg/L (15.2–188.0 mg/L)] was higher than the reference range (0–10 mg/L); and the ESR was higher than the reference range (0–20 mm/h) in five cases [median =79 mm/h (25–132 mm/h)]. Imaging studies demonstrated conclusive signs of pulmonary infection in all of seven patients; and in two cases, concurrent bilateral pleural effusion was shown.
Discussion
Allo-HSCT is a procedure with a high-risk for infections (11). Before transplantation, recipients were prepared with myeloablative conditioning regimens that deplete leukocytes. The occurrence of agranulocytosis for 2–3 weeks from d 1 usually may cause a more severe infection if it occurs during this period (12). We identified seven cases of pulmonary infection in which a diagnosis was determined based on clinical symptoms, a biochemical assay, microbial culture and diagnostic imaging results, which accounted for a 2.21% incidence rate of pre-transplant pulmonary infection. Specifically, the following clinical symptoms were found among these cases: (I) patients showed clinical symptoms of infection; (II) chest radiography findings were suggestive of pulmonary infection; (III) seven patients had a CRP level above the reference range and five had an increased ESR; (IV) the 1,3-β-D-glucan and Aspergillus galactomannan levels were within normal ranges; (V) oropharyngeal swab results were negative; (VI) blood culture showed no growth of bacteria, anaerobic bacteria, and fungi; and (VII) stool culture findings did not reveal Salmonella, Shigella, and fungi. The negative microbiological cultures may result from aggressive prophylaxis and anti-infective therapy with antibiotics. Furthermore, a sterile laminar-flow environment, the presence of agranulocytosis, medication for infection and GVHD may all have an impact on test results and mislead physicians (13); thus, the importance of a timely diagnosis of infection cannot be over-emphasized (14).
Our experience with these patients suggested that the CRP level and chest radiography findings might be useful for monitoring the development of pre-transplant pulmonary infection in HLFU. This was based on our observation that, in most of times, a pre-transplant pulmonary infection became suspected when the patient presented with a fever, and was subsequently confirmed by using bedside chest radiography and laboratory investigations. Although HRCT can detect infective lesions <30 mm (15), and is thus more effective and accurate for detecting pulmonary infection, the examination cannot be performed in a HLFU where patients are subjected to conditioning. We speculated that the actual incidence rate of pulmonary infection developed in HLFU might be higher than as noted in our study (2.21%).
A previous study reported that the incidence of non-relapse mortality after haploidentical HSCT was 18.1% (16). In our study, all patients who developed pre-transplant pulmonary infection in HLFU underwent HLA haploidentical HSCT. The transplantation was conducted in the seven patients as scheduled when their temperature and symptoms were considered acceptable after aggressive antimicrobial therapy. There was a significantly higher risk of death early after HCST among patients who developed pulmonary infection with bilateral pleural effusion prior to graft infusion of stem cells; as noted in the present study, two patients died of infection that was exacerbated by multiple organ failure within 3 post-transplant months. Compared with the other patients, those who developed pre-transplant pulmonary infection in the sterile laminar flow unit were more susceptible to pulmonary infection after transplantation, although the long-term OS was not affected. Additionally, of the two recipients who died of pulmonary infection, one received allograft from a female cousin. Regarding the five survival recipients who had pulmonary infection, their donors were all men (father or son), which is consistent with the conclusion of our institution that cases with male donors are associated with a lower transplantation-related mortality and higher survival rate (7). This suggested that use of male donors may decrease the mortality of recipients with pre-transplant pulmonary infection.
CD4CD45RA T-cells help suppress the immune response, whereas CD4CD45RO T cells facilitate induction; they are both of vital importance in the development, functional regulation, and signal transduction of lymphocytes (17). Our data demonstrated that compared with patients who did not develop any pre-transplant pulmonary infection, the group with pulmonary infection in HLFU had higher level of CD4CD45RO+CD45RA− (P=0.008) and lower level of CD4CD45RO−CD45RA+ (P=0.033), which implies that early immune reconstitution may be somehow influenced by the infection. This may have been a factor contributing to post-transplant relapse of infection in the five surviving patients who suffered pulmonary infection in HLFU (3 cases had infection in the first month, and 2 cases in the second month after allo-HSCT). No obvious effect of CD4CD45RO+CD45RA on immune reconstitution was noted in the third and fourth months after transplantation.
In conclusion, development of pre-transplant pulmonary infection in HLFU among patients with hematological malignancies scheduled for HLA-haploidentical HSCT is associated with an increased risk of recurrent pulmonary infection in the early period after transplantation; however, the long-term survival rate, is not associated with the infection status.
Acknowledgements
We thank the staff from Department of Hematology, Peking University People’s Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethics statement: This study was approved by the Ethics Committee of Peking University People’s Hospital (No. [2012]26), and it was conducted in accordance with the Declaration of Helsinki. All subjects gave written informed consent before participating in the study.
References
- Chang YJ, Huang XJ. Haploidentical hematopoietic stem cell transplantation with unmanipulated granulocyte colony stimulating factor mobilized marrow and blood grafts. Curr Opin Hematol 2012;19:454-61. [Crossref] [PubMed]
- Martín-Peña A, Aguilar-Guisado M, Espigado I, et al. Prospective study of infectious complications in allogeneic hematopoietic stem cell transplant recipients. Clin Transplant 2011;25:468-74. [Crossref] [PubMed]
- Aguilar-Guisado M, Jiménez-Jambrina M, Espigado I, et al. Pneumonia in allogeneic stem cell transplantation recipients: a multicenter prospective study. Clin Transplant 2011;25:E629-38. [Crossref] [PubMed]
- Wang CY, Ren HY, Qiu ZX, et al. Prognostic implications of hematopoietic cell transplantation-specific comorbidity index on non-relapse mortality and overall survival after allogeneic hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue Za Zhi 2013;34:659-63. [PubMed]
- Lu DP, Dong L, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006;107:3065-73. [Crossref] [PubMed]
- Han W, Huang XJ, Liu KY, et al. The efficacy and safety of mismatched hematopoietic stem cell transplantation for treatment of severe aplastic anemia. Zhonghua Nei Ke Za Zhi 2011;50:287-90. [PubMed]
- Wang Y, Chang YJ, Xu LP, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood 2014;124:843-50. [Crossref] [PubMed]
- Liu DH, Chen SS, Sun YQ, et al. Clinical characteristics of late-onset severe pneumonia after allogeneic hematopoietic stem cell transplantation. Zhonghua Nei Ke Za Zhi 2013;52:819-23. [PubMed]
- Ministry of Health of the People’s Republic of China. Diagnostic criteria for nosocomial infections. Chin Med J 2001;81:314-20.
- Editorial Committee of Chinese Journal of Internal Medicine. The diagnostic criteria and treatment principles of Invasive pulmonary fungal infection(Draft). Chinese Journal of Internal Medicine 2006;45:697-700.
- Libbrecht C, Goutagny MP, Bacchetta J, et al. Impact of a change in protected environment on the occurrence of severe bacterial and fungal infections in children undergoing hematopoietic stem cell transplantation. Eur J Haematol 2016;97:70-7. [Crossref] [PubMed]
- Han TT, Huang XJ, Liu KY, et al. Blood stream infections during agranulocytosis period after hematopoietic stem cell transplantation in one single center. Zhonghua Nei Ke Za Zhi 2011;50:654-8. [PubMed]
- Sjøqvist C, Snarski E. Inflammatory markers in patients after hematopoietic stem cell transplantation. Arch Immunol Ther Exp (Warsz) 2013;61:301-7. [Crossref] [PubMed]
- Kambham N, Higgins JP, Sundram U, et al. Hematopoietic stem cell transplantation: graft versus host disease and pathology of gastrointestinal tract, liver, and lung. Adv Anat Pathol 2014;21:301-20. [Crossref] [PubMed]
- Tsujimoto N, Saraya T, Kikuchi K, et al. High-resolution CT findings of patients with pulmonary nocardiosis. J Thorac Dis 2012;4:577-82. [PubMed]
- Wang Y, Liu DH, Liu KY, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 2013;119:978-85. [Crossref] [PubMed]
- Machura E, Mazur B, Pieniazek W, et al. Expression of naive/memory (CD45RA/CD45RO) markers by peripheral blood CD4+ and CD8 + T cells in children with asthma. Arch Immunol Ther Exp (Warsz) 2008;56:55-62. [Crossref] [PubMed]