Clinicopathological characteristics and prognosis of non-lepidic invasive adenocarcinoma presenting as ground glass opacity nodule
Introduction
Adenocarcinoma is the most common histologic type of non-small cell lung cancer (1). Recently, the use of chest computed tomography (CT) for lung cancer screening and early-stage lung cancer detection has increased (2). The presence of air density areas known as ground glass opacity (GGO) nodules has been used as a screening indicator for lung cancer. With advances in technology, detection of GGO has been increased remarkably, especially in Korea. Several studies have shown that persistent GGO nodules on CT had a high risk of malignancy (3,4), and almost of these nodules were adenocarcinoma, which is characterized by histologic heterogeneity. The International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS) proposed new classification of adenocarcinoma in 2011 (5), and in 2015 the classification was adopted in World Health Organization (WHO) classification of Tumors of the Lung, Pleura, Thymus and Heart 4th edition (6). With these changes, malignant nodules presenting as GGO are regarded as low-grade malignancies with three subtypes: adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA) or lepidic adenocarcinoma. This group of tumors are correlated with a favorable prognosis after surgical resection. However, evidence suggests that other more invasive, non-lepidic growth patterns of adenocarcinoma such as acinar and papillary have presented as GGO nodules (7-11).
In many institutions, surgical treatment of lung cancer is planned by radiologic findings because histomorphologic subtypes are only confirmed postoperatively. Within our institution, GGO is regarded as a suitable candidate for curative treatment by means of sublobar resection. However, with recent evidence that some GGOs may be associated with non-lepidic invasive adenocarcinoma, there is likely a percentage of tumors presenting as GGOs in which sublobar resection may not represent the best treatment of choice. Therefore, it is of interest to assess the tumor characteristics and prognosis of non-lepidic invasive adenocarcinoma presenting as GGO nodules.
We herein report the clinicopathological characteristics and survival outcomes from non-lepidic invasive adenocarcinoma tumors which were GGO-predominant nodules on chest CT. These results were compared with tumors that were solid-predominant on chest CT. The aim of this study was to clarify the clinical significance of lung adenocarcinoma presenting with GGO, specifically with regard to prognosis.
Methods
Patients
This retrospective study was approved by the institutional Review Board of Seoul St. Mary’s Hospital (The Catholic University of Korea, ID: KC16RISI0263). A total of 857 consecutive patients at Seoul St. Mary’s Hospital in Korea were diagnosed with non-small cell lung cancer and underwent surgical resection between January 2010 and December 2014. Of this population, 334 patients were diagnosed as pathologic stage I adenocarcinoma and underwent anatomical complete resection via lobectomy or bilobectomy with mediastinal lymph node dissection. Disease staging was based on the 7th American Joint Committee on Cancer (AJCC) guidelines (12). Complete resection was defined as an absence of residual cancer both macroscopically and microscopically. Among 334 patients with stage I adenocarcinoma, tumor sizes of 281 patients were ≤3 cm. We excluded tumors classified according to the 2015 WHO classification as AIS (8 patients), MIA (48 patients) or lepidic adenocarcinoma (57 patients). The remaining 168 patients were divided into two groups according to the extent of radiologic findings of GGO on chest CT preoperatively: GGO-predominant invasive adenocarcinoma (Group A) and solid-predominant invasive adenocarcinoma (Group B). Clinicopathological features and survival were compared among the two groups.
Radiologic evaluation
Primary lesions were evaluated using thin-section CT images. All chest CT scans were obtained at full inspiration and were retrospectively examined for GGO nodules. GGO is defined on a CT scan by hazy increased opacities in the lung parenchyma, with preservation of bronchial structures and vascular margins (13). The diameter of the tumor (T) was defined as the largest axial diameter of the lesion on the lung window setting. The diameter of consolidation (C) on the axial image on the lung window setting was also measured, where consolidation was defined as an area of increased opacification which completely obscured underlying bronchial structures and vascular markings. GGO-predominant tumors were those with a C/T ratio <0.5 and solid-predominant tumors were those with a C/T ratio ≥0.5. Each lung nodule on preoperative CT scans was reviewed blindly by two thoracic surgeons.
Histologic evaluation
All clinical specimens were examined by pathology specialists, whose observations were recorded. Each tumor was reviewed for size, location, differentiation, lymph node status, pleural invasion, lymphatic invasion, and vascular invasion. To describe histomorphologic patterns of tumors, the occupancy ratio of each component (lepidic, acinar, papillary, micropapillary, and solid) in the total tumor area was measured and recorded semiquantitatively in 5% increments, according to the 2015 WHO classification of lung tumors (6). AIS and MIA defined as small (≤3 cm), and solitary adenocarcinomas that consisted of lepidic growth pattern without invasion (AIS) or with ≤5 mm invasion (MIA). Invasive adenocarcinomas classified into several subtypes (acinar adenocarcinoma, papillary adenocarcinoma, micropapillary adenocarcinoma, lepidic adenocarcinoma, etc.). Non-lepidic invasive adenocarcinoma defined as invasive adenocarcinomas except lepidic adenocarcinoma.
Statistical analysis
Clinicopathological factors were for each group were analyzed with the Student’s t-test or the Wilcoxon rank-sum test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. Data for the interval between surgical resection and last follow-up visit were analyzed via the Kaplan-Meier method using confirmed recurrences/deaths to calculate recurrence-free survival (RFS) and overall survival. Survival of each group was compared by log-rank test and the Cox proportional hazards model of multivariate analysis was engaged to determine risk of recurrence. A value of P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
Results
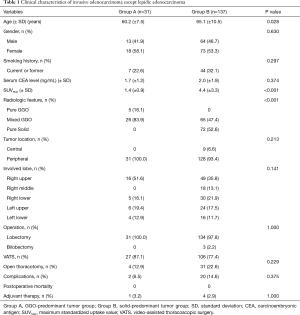
A comparison of clinicopathological characteristics between groups A and B is shown in Table 1. Of the 168 subjects, a total of 31 had nodules classified as GGO-predominant (Group A) and the remaining 137 had nodules classified as solid-predominant (Group B). Of the 31 subjects in the GGO-predominant group, the mean age was 60.2 years (SD, 7.5) and 58.1% were women. Smoking history was present in 7 (22.6%) of the subjects. The mean serum carcinoembryonic antigen (CEA) level was 1.7 ng/mL (SD, 1.2). Of this group 5 subjects (16.1%) had pure GGO nodules and 26 subjects (83.9%) had mixed GGO nodules. All tumors were peripherally located and treated with lobectomy; video-assisted thoracoscopic surgery (VATS) was used in 87.1% of all cases. In the 137 subjects assigned to the solid-predominant group, the mean age was 65.1 (SD, 10.5), and 53.3% were women. Within this group, 32.1% were current or former smokers. The mean serum CEA level was 2.0 ng/mL (SD, 1.9). A total of 65 subjects (47.4%) had mixed GGO and 72 patients (52.6%) had pure solid nodules. Of the total 137 subjects, 128 (93.4%) had peripherally located tumors, 3 underwent bilobectomy, and VATS was used in 77.4% of all cases. The mean maximum standardized uptake value (SUVmax) of fluorodeoxyglucose on PET scanning was lower in Group A than Group B [1.4 (±0.9) vs. 4.4 (±3.3), respectively; P<0.001]. Otherwise, there were no significant differences between groups with regard to sex, smoking status, mean serum CEA, involved lobes, complication rate, postoperative mortality, or frequency of adjuvant therapy.
Full table
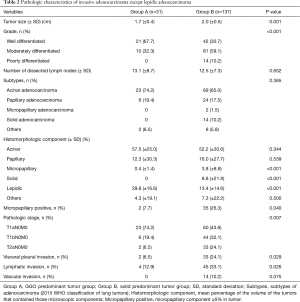
Pathologic characteristics of the tumors in both groups are shown in Table 2. The mean tumor size was significantly less in Group A than in Group B (1.7 vs. 2.0 cm, P=0.001). More moderately- to poorly-differentiated tumors were present in Group B (P<0.001). The mean total number of lymph nodes removed per patient was 13.1 (±8.7) and 12.5 (±7.3), respectively (P=0.652). The distribution of subtypes of adenocarcinoma (acinar, papillary, micropapillary, solid, lepidic, or other types) was not different between the two groups (P=0.365). We compared the mean percentages of the volume of the five histomorphologic components (acinar, papillary, micropapillary, solid, and lepidic) in the tumors. Acinar and papillary tumor components were present in similar percentages in both groups. However, micropapillary and solid components were present more frequently in Group B, and a lepidic component was present more frequently in Group A. Specifically, the incidence of a micropapillary component ≥5% within the tumors was 7.7% (2 cases) in Group A and 26.3% (35 patients) in Group B (P=0.040). The distribution of pathologic stage between the groups differed, where T1bN0M0 and T2aN0M0 were more frequently diagnosed in Group B (P=0.007). Solid-predominant tumors had pleural invasion, lymphatic invasion, and vascular invasion more frequently than GGO-predominant tumors (P=0.029, 0.026, 0.075).
Full table
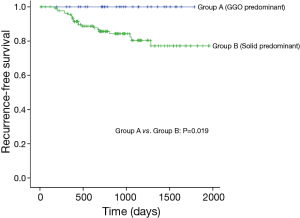
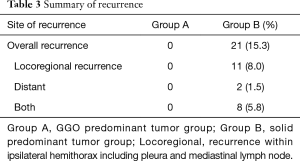
Median follow-up time for all patients was 875 days (range, 12–1,957 days) and recurrences were noted in 21 patients (Table 3). None of the patients with GGO-predominant tumors experienced a recurrence (3-year RFS, 100%). Of the patients with solid-predominant tumors, 80.4% had a 3-year RFS (Figure 1).
Full table
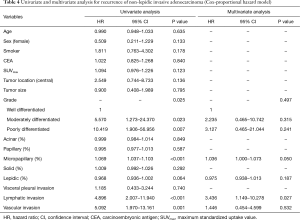
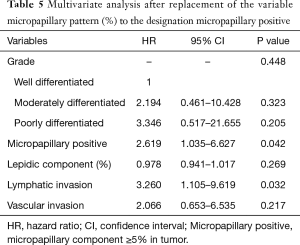
Table 4 shows univariate and multivariate analysis using the Cox proportional hazards model to determine factors associated with recurrence of ≤3 cm non-lepidic invasive adenocarcinoma presenting as GGO nodules on chest CT. Multivariate analysis with grade of tumor differentiation, percentage of micropapillary pattern in the tumor, percentage of lepidic pattern in the tumor, lymphatic invasion, and vascular invasion indicated that micropapillary pattern [hazard ratio (HR) =1.036, 95% confidence interval (CI) 1.000–1.073; P=0.050] and lymphatic invasion (HR =3.436; 95% CI, 1.149–10.278; P=0.027) were significant risk factors for recurrence. In multivariate analysis, replacement of percentages of the micropapillary component of the tumor (continuous variable) with the designation of micropapillary positive (categorical variable, micropapillary component ≥5% in tumor) resulted in micropapillary positive (HR =2.619; 95% CI, 1.035–6.627; P=0.042) and lymphatic invasion (HR =3.260; 95% CI, 1.105–9.619; P=0.032) as statistically significant risk factors for recurrence (Table 5). Overall, risk factors for recurrence were present more frequently in Group B, the solid-predominant tumors.
Full table
Full table
Discussion
In the present study, our aim was to elucidate the prognosis of invasive adenocarcinoma presenting clinically as GGO nodules on chest CT. Prior studies have shown outcomes of 100% RFS after surgical resection for AIS and MIA, and among the five subtypes of adenocarcinoma, lepidic adenocarcinoma showed the most favorable prognosis (14,15). Some reports also determined that the degree of lepidic pattern in a tumor was related to disease prognosis, where greater than 50% lepidic pattern was a favorable prognostic indicator in cases of invasive adenocarcinoma (16-18). Generally, GGO on a chest CT is considered a lepidic component suggestive of AIS, MIA, or lepidic adenocarcinoma, which are grouped along a continuum as minimally invasive tumors. As a result, many surgeons choose sublobar resection (wedge resection or segmentectomy) for curative treatment of GGO nodules. However, GGO nodules do not always represent AIS, MIA or lepidic adenocarcinoma. Indeed, in some cases, GGO nodules have been associated with non-lepidic invasive adenocarcinoma. For those reasons, recent studies have been conducted to ascertain the predictive factors for the presence of invasive adenocarcinoma when GGO nodules are present (19-21).
We defined GGO-predominant nodules as those with a C/T ratio <0.5 and solid-predominant nodules as those with a C/T ratio ≥0.5. These criteria were adopted based on the National Comprehensive Cancer Network guidelines for non-small cell lung cancer (Version 4.2016) which state that segmentectomy or wedge resection is appropriate in selected patients. One of those indications is for nodules with greater than 50% GGO appearance on CT. Moreover, two ongoing studies (JCOG 0802, JCOG 0804) have also adopted C/T ratio to select sublobar resection (14,22). Clinically, it is practical for thoracic surgeons to use the C/T ratio to classify nodules on CT as GGO-predominant or solid-predominant.
In this study, we found that a micropapillary component and lymphatic invasion are the significant risk factors for recurrence in non-lepidic invasive adenocarcinoma of ≤3 cm. Solid-predominant nodules (Group B) had more risk factors for recurrence when compared with GGO-predominant nodules (Group A). The incidence of micropapillary positive tumors and the presence of lymphatic invasion within the tumor were greater in Group B than Group A. In addition, SUV max, which is a known prognostic factor in non-small cell lung cancer (23,24), was greater in Group B than Group A. Other factors such as larger tumor size, poor tumor differentiation, and visceral pleural invasion also supported poorer prognosis of Group B when compared with Group A. Interestingly, the 3-year RFS for individuals with GGO-predominant nodules (Group A) was 100% in this study, considering all cases were those of known invasive adenocarcinoma. We therefore concluded that GGO nodules have a lesser malignant potential when compared with solid nodules on chest CT, despite the fact that GGO nodules are associated with non-lepidic invasive adenocarcinoma.
We analyzed the five major histomorphologic growth patterns of adenocarcinoma (acinar, papillary, micropapillary, solid and lepidic component) and compared the distribution of these growth patterns between the two study groups. Although the distribution of WHO’s classifications of adenocarcinoma (acinar adenocarcinoma, papillary adenocarcinoma, micropapillary adenocarcinoma, and solid adenocarcinoma) were similar between both groups, the mean percentages of each of the growth patterns in the tumors were significantly different between the two groups, especially with respect to the presence of micropapillary, solid and lepidic components. Specifically, the mean percentages of micropapillary and solid components were higher in Group B. Conversely, the mean percentage of a lepidic component was lower in Group B. These findings are congruent with prior studies which have demonstrated that micropapillary and solid tumor components have a higher malignant potential than acinar, papillary, and lepidic components (14,25). Therefore, from a histomorphologic perspective, solid-predominant tumors (Group B) have a greater malignant potential than GGO-predominant tumors (Group A). In the case of pure GGOs (n=5), acinar, papillary, and lepidic components were only present in the tumor, and overall in Group A, micropapillary and solid components were seldom present. We adopted the presence of a micropapillary component as an independent variable, because some studies reported that tumor prognosis changes when a small amount of micropapillary component (micropapillary ≥5%) is present (14,26,27). In our study, micropapillary positive designation was a significant prognostic factor for recurrence and was more frequently present in Group B.
Historically, GGO may be a transient finding, and when persistent is considered to represent a precancerous growth that may progress down the spectrum of lepidic adenocarcinoma. However, on final pathology after surgical resection, GGO may at times be diagnosed as non-lepidic invasive adenocarcinoma. Our study showed that non-lepidic invasive adenocarcinoma presenting as GGO had an excellent prognosis (100% 3-year RFS). In other words, the presence of GGO on a chest CT is a good prognostic factor for lung cancer irrespective of the tumor’s histomorphologic classification. Therefore, in preoperative evaluation, chest CT considering GGO as a low-grade malignant tumor may be reasonable.
In this study, we evaluated RFS instead of overall survival because in the case of stage I disease, more patients die from other causes than from the cancer during the follow-up period (14). Also, RFS is a more accurate measurement of survival analysis, since it reflects the biological behavior of the cancer rather than death due to unrelated factors.
This study has several limitations that should be considered. First, we used a retrospective study design. Second, we obtained the data from a single institution and the number of cases was relatively small. For instance, the number of patients with GGO-predominant nodules was 31, therefore a 100% 3-year RFS may not be representative of the true survival outcome when extrapolated to a larger population. However, our results indicate a better prognostic outcome of GGO-predominant nodules when compared with solid-predominant nodules, in a manner that was statistically significant. Of course, more accurate results could be obtained if the analysis and comparison were made with a larger patient sample. Finally, we used recent data (2010 to 2014), which limited the amount of available follow-up information and permitted evaluation of only a 3-year survival outcome. However, most cases of recurrence of lung cancer occur within 2 years (28) and therefore the use of a 3-year RFS as an outcome measure is suitable for the determination of prognosis.
In conclusion, non-lepidic invasive adenocarcinoma presenting as a GGO-predominant nodule has fewer risk factors for recurrence and a better prognosis than non-lepidic invasive adenocarcinoma presenting as a solid-predominant nodule. Therefore, limited resection in the case of all of GGO-predominant nodules, irrespective of histomorphologic patterns of adenocarcinoma, may be a reasonable course of action. Further studies that include data from larger homogenous sample sizes may provide more accurate results.
Acknowledgements
This article has been edited by native English-speaking experts of BioMed Proofreading, LLC.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the institutional Review Board of Seoul St. Mary’s Hospital (The Catholic University of Korea, ID: KC16RISI0263).
References
- Parkin DM, Ferlay J, Curado MP, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer 2010;127:2918-27. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Kim HY, Shim YM, Lee KS, et al. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology 2007;245:267-75. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Wilshire CL, Louie BE, Manning KA, et al. Radiologic Evaluation of Small Lepidic Adenocarcinomas to Guide Decision Making in Surgical Resection. Ann Thorac Surg 2015;100:979-88. [Crossref] [PubMed]
- Sim HJ, Choi SH, Chae EJ, et al. Surgical management of pulmonary adenocarcinoma presenting as a pure ground-glass nodule. Eur J Cardiothorac Surg 2014;46:632-6. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Cho H, Lee HY, Kim J, et al. Pure ground glass nodular adenocarcinomas: Are preoperative positron emission tomography/computed tomography and brain magnetic resonance imaging useful or necessary? J Thorac Cardiovasc Surg 2015;150:514-20. [Crossref] [PubMed]
- Duann CW, Hung JJ, Hsu PK, et al. Surgical outcomes in lung cancer presenting as ground-glass opacities of 3 cm or less: a review of 5 years' experience. J Chin Med Assoc 2013;7:693-7. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York: Springer, 2010.
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Moon Y, Sung SW, Lee KY, et al. The importance of the lepidic component as a prognostic factor in stage I pulmonary adenocarcinoma. World J Surg Oncol 2016;14:37. [Crossref] [PubMed]
- Moon Y, Kim KS, Sung SW, et al. Correlation of histological components with tumor invasion in pulmonary adenocarcinoma. World J Surg Oncol 2014;12:388. [Crossref] [PubMed]
- Son JY, Lee HY, Kim JH, et al. Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: the added value of using iodine mapping. Eur Radiol 2016;26:43-54. [Crossref] [PubMed]
- Liu LH, Liu M, Wei R, et al. CT findings of persistent pure ground glass opacity: can we predict the invasiveness? Asian Pac J Cancer Prev 2015;16:1925-8. [Crossref] [PubMed]
- Jin X, Zhao SH, Gao J, et al. CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground-glass opacity. Eur Radiol 2015;25:2532-40. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6-12. [Crossref] [PubMed]
- Li M, Wu N, Zheng R, et al. Primary tumor PET/CT [18F]FDG uptake is an independent predictive factor for regional lymph node metastasis in patients with non-small cell lung cancer. Cancer Imaging 2013;12:566-72. [Crossref] [PubMed]
- Sasada S, Nakayama H, Miyata Y, et al. Comparison of malignant grade between pure and partially invasive types of early lung adenocarcinoma. Ann Thorac Surg 2015;99:956-60. [Crossref] [PubMed]
- Chao L, Yi-Sheng H, Yu C, et al. Relevance of EGFR mutation with micropapillary pattern according to the novel IASLC/ATS/ERS lung adenocarcinoma classification and correlation with prognosis in Chinese patients. Lung Cancer 2014;86:164-9. [Crossref] [PubMed]
- Sumiyoshi S, Yoshizawa A, Sonobe M, et al. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer 2013;81:53-9. [Crossref] [PubMed]
- Tremblay L, Deslauriers J. What is the most practical, optimal, and cost effective method for performing follow-up after lung cancer surgery, and by whom should it be done? Thorac Surg Clin 2013;23:429-36. [Crossref] [PubMed]