Video-assisted thoracic surgery compared with posterolateral thoracotomy for mediastinal bronchogenic cysts in adult patients
Introduction
Bronchogenic cyst (BC) is a rare congenital malformation, which arises from abnormal budding of the ventral diverticulum of the foregut or the tracheobronchial tree between the 26th and 40th days of gestation (1,2). Depending on the timing, the said abnormality may be located in the mediastinum or the pulmonary parenchyma but rarely in the extra-thoracic sites (2,3). Although BCs are the most common primary cysts found in the mediastinum and represent 18% of all primary mediastinal malformations, the exact incidence of Mediastinal bronchogenic cysts (MBCs) is unknown because most patients are asymptomatic (1,4,5). However, more and more patients were detected because of the advanced diagnostic equipment and the increasing application of routine medical examinations in recent years.
For adult patients with MBC, surgical resection is generally performed through posterolateral thoracotomy (PLT). With the recent advances in endoscopic instruments and operative techniques, video-assisted thoracic surgery (VATS) resection of MBC has been used with increasing frequency since it was first performed by Mouroux and associates in 1991 (6); it may become a potentially new surgical choice for the treatment of MBCs. However, limited studies have been published to compare the results of VATS versus PLT in adult patients with MBC, thus, uncertainty remains as to whether VATS can be an alternative to PLT for the treatment of MBCs. Thus, we retrospectively reviewed the data of patients with MBCs to reveal whether VATS was superior to PLT or not when concerning the effectiveness and safety in treating MBCs.
Methods
Data collection
Patients with MBCs who underwent surgical resection between August 2005 and December 2015 were identified from the electronic database of the Department of Thoracic Surgery, West China Hospital, Sichuan University. The clinical history of these patients was reviewed including the following items: patient demographic characteristics [age, gender, body mass index (BMI) at the time of operation, preoperative symptoms, and anatomical location of the cyst], intraoperative findings (maximum diameter of cysts, operative time, intra-operative blood loss, reason of conversion to thoracotomy, pleural atresia, incomplete resection, and surgery-related complications), postoperative outcomes (the duration of intensive care unit (ICU) stay, duration of chest drainage, pleural drainage of the first three days after surgery, length of postoperative hospital stay, and incidence of postoperative complications), and follow up information.
All the patients had routine preoperative examination, including a complete blood test, a computed tomography (CT) or/and magnetic resonance imaging (MRI) scan of the chest. The operation was performed by different surgeons (no trainees) of our department, while the surgical approach was decided according to the surgeon’s training experience. Some of the surgeons in our department prefer to perform open surgery, while the other prefer VATS. Informed consent of the operation was obtained from all patients before surgery. Postoperative follow-up was conducted through phone call to reveal the release of preoperative symptoms, recurrence and patient survival. The protocol of the study was approved by the Institutional Review Board (IRB) of West China Hospital (NO. 2016-168); informed consent was waived for this retrospective research.
Surgical procedure
Both PLT and VATS procedures were carried out with double-lumen endotracheal intubation. For patients who underwent PLT, the incision was made in the fourth to sixth intercostal space, depending on the anatomic site of the cyst. On the other hand, a three-portal procedure was applied to patients who underwent VATS. The camera port (1 cm) was placed in the sixth or seventh intercostal space at the mid-axillary line. The other two ports were made according to the site of the cysts. Cysts were excised with sharp and blunt dissection using energy devices and endoscopic scissors. When excising the cyst, we tried to maintain the integrality of the cyst wall in order to achieve a complete removal. However, when the cyst was too large and obscuring surgical view, we first aspirated the cystic contents. At the end of surgery, one drainage tube was placed under direct vision. Postoperative pain management was achieved through patient controlled analgesia. The chest tube was removed given that the pleural drainage was less than 200 mL per day.
Data were analyzed using SPSS version 16.0 (SPSS Inc., Chicago, Ill, USA). Continuous variables with normal distribution were presented as mean ± standard deviation (SD), otherwise were presented as median and range. The differences between each group were assessed by a two-tailed Students’ t-test or Mann-Whitney U-test. Dichotomous variables were presented as numbers and percentages and were analyzed using Pearson’s chi-square test or Fisher exact test. A P value less than 0.05 was denoted as statistically significant.
Results
A total of 107 patients with MBC underwent surgical resection from August 2005 to December 2015. Patients who underwent median sternotomy (N=5) or video-assisted minithoracotomy (N=3) were excluded. The remaining 99 patients were observed, 65 of whom underwent VATS procedure while 34 underwent PLT procedure.
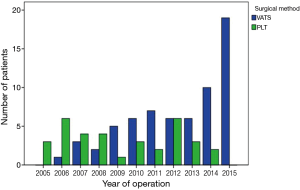
The proportion of VATS procedure for MBC resection gradually increased during that period, from none VATS cystectomy in 2005 to none PLT cystectomy in 2015 (Figure 1). The demographic characteristics of all patients undergoing PLT and VATS procedure are shown in Table 1. There were no statistical differences between the two groups in age, gender, and BMI. The location of the lesions was variable, and was classified according to a 3-compartment model (7). The distribution of the lesions was approximately similar among the two groups. A total of 52 patients (52.52%) exhibited different symptoms (including 9 patients having more than one symptom). Having the most complaints, cough was exhibited in 23 patients (VATS: 16 and PLT: 7), chest pain in 17 patients (VATS: 10 and PLT: 7), dyspnea in 14 patients (VATS: 8 and PLT: 6), hoarseness in five patients (VATS: 4 and PLT: 1), and dysphagia in two patients (VATS: 1 and PLT: 1). Forty-seven patients (47.48%) (VATS: 32 and PLT: 15) were asymptomatic; the bronchogenic cyst was incidentally discovered by routine examination, whereas no significant differences were observed regarding the incidence of preoperative symptoms between the two groups.
Full table
All patients underwent CT scans, wherein 47 cysts (VATS: 30 and PLT: 17) manifested as water-attenuation masses, while the remaining 52 (VATS: 35 and PLT: 17) manifested as soft-tissue-attenuation masses. Ten patients underwent both CT and MRI scans. On T1-weighted images, six cysts were slightly hyperintense or isointense to skeletal muscle, while the other four cysts were isointense to cerebrospinal fluid (CSF). On T2-weighted MRI, seven cysts were isointense to CSF, while three cysts were much hypointense to CSF. None of the cysts showed enhancement in either contrast-enhanced CT or gadolinium enhanced MRI scan.
The intraoperative findings and perioperative outcomes of the patient in the VATS and PLT groups are listed in Table 2. There were no statistically significant differences between the PLT and VATS groups with regard to the maximum diameter of cysts, pleural atresia, surgery-related complications, and duration of intensive care unit stay. However, VATS was associated with shorter operative time (108.77±47.81 vs. 144.62±55.16, P=0.001), shorter duration of chest drainage (2.52±1.29 vs. 3.71±1.55, P<0.001), shorter postoperative hospital stay (4.94±2.01 vs. 8.64±5.52 P=0.001), less intra-operative blood loss (median: 20 vs. 100, P<0.001), and less pleural drainage of the first three days after surgery (median: 240 vs. 400, P=0.002) compared to the PLT group.
Full table
Postoperative complications included one patient with pulmonary infection and one patient with postoperative bleeding who experienced re-operation in the VATS group, and two patients with chylothorax in the PLT group. However, there was no significant difference of the postoperative complications between the two groups. Five cysts (VATS: 2 patients, PLT: 3 patients) were removed incompletely due to severe adhesion to nearby vital structures including thoracic aorta (2 patients), trachea (1 patient) and esophagus (2 patients). Part of the cyst wall was left in place and the mucosa was obliterated by electrocautery to prevent recurrence. There was no significant difference of incomplete resection between these two groups. In VATS group, one patient was converted to PLT due to left pulmonary artery injury and uncontrollable bleeding.
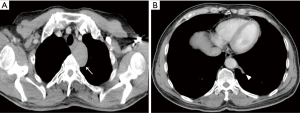
In the VATS group, one patient was observed to have double lesions in the anterosuperior mediastinum and the left lower lobe (Figure 2). Mediastinal cyst excision and mass excision of the sequestered lung tissue was performed simultaneously. The pulmonary lesions were confirmed postoperatively as extralobar pulmonary sequestration (PS) by pathological examination. No perioperative deaths occurred in either group.
In February 2016, telephone follow-ups were conducted. Seven patients (5 cases in VATS group and 2 cases in PLT group) did not respond to the follow-up while the remaining patients responded at periods ranging from 2 to 129 months with a median of 48 months. During the follow-up period, none of the patients had recurrence of the MBC and death attributing to MBC.
Discussion
BCs are mainly unilocular and the classical histological features consist of a lining of respiratory epithelium associated with a wall cartilage, containing glands, and smooth-muscle elements, which is responsible for the contents of cyst (5,8). We reviewed the literature and found that there was no study on the underlying pathogenesis especially the molecular mechanism. BC is a kind of bronchopulmonary foregut malformations (BFM), which is correlated with defective budding, differentiation, and separation of the primitive foregut; in addition to BC, BFM encompasses intralobar PS, extralobar PS, bronchial atresia, congenital pulmonary airway malformation, and congenital lobar hyperinflation (9). Various types of BFM may occur in combination with each other (10). In 1982, Croyle and colleagues first reported intralobar PS and MBC in the same patient (11). Since then, some case reports have been published to report coinstantaneous intralobar or extralobar PS and MBC in the same patient (12,13). In our series, one patient had co-existing MBC and extralobar PS, and simultaneous excision was conducted.
MBCs have a wide spectrum of clinical manifestations that can be completely asymptomatic or accompanied by complications. The incidence of clinical symptoms in patients with MBC varied from 9% to 67% (4,6). The most common symptoms are chest pain, dyspnea, cough and fever. However, these symptoms are not specific making it hard to proceed with the diagnosis preoperatively with only a history of the disease. For the current study, symptoms were present in 52.52% patients. The proportion of symptomatic patients is higher compared with that recorded in other published reports.
CT and MRI are important examinations used to reveal the relationship between the cyst and the surrounding vital structures, as well as the information about the size and shape of the cyst (5). However, the imaging manifestation was variable. McAdams and colleagues (10) retrospectively reviewed imaging features about a large series of 58 mediastinal and 10 extra mediastinal bronchogenic cysts. They concluded that variability in the contents of cyst were likely responsible for the difference in imaging manifestations on CT and MRI scans. In our study, cysts among 52.53% of the patients were shown as soft-tissue-like on CT images. In these patients, although the contents of the cyst were not chemically analyzed, it ranged from hemorrhagic fluid to a very viscous, mucoid material. We also think that these contents might contribute to the increased attenuation on CT scans. Four cysts were projected as water-like on MRI while they were all seen as soft-tissue-like on CT images, which demonstrated that MRI may be useful for differentiating MBC from mediastinal neoplasia when CT scans manifest as soft-tissue-like lesions.
For patients exhibiting symptoms, surgical resection is the preferred treatment modality (14). Other treatment modalities, such as robotic thoracic surgery and endobronchial ultrasound-guided transbronchial needle aspiration (15,16), are still in the developmental stages, and follow-up is still needed to verify the long-term efficacy. However, there is still controversy in choosing an appropriate timing of surgery for asymptomatic patients (17). Some authors suggested that surgical resection should be conducted only in cases of suspicion of malignant neoplasm (18,19). However, in the past decades, some studies have shown that asymptomatic patients can develop symptoms during the observation period, and some complications will occur, such as superior vena cava obstruction, infection, pneumothorax, pleural effusion, and arrhythmia even before the onset of symptoms (6,17,20). The potential for malignant changes has been reported in patients with BC (2,21-23). In recent years, more and more patients with BC are being diagnosed antenatally with the advent of antenatal diagnosis, which has provided the possibility of studying the natural history of BC (17,24-27). Yan and colleagues (24), who retrospectively reviewed clinical features of BC diagnosed antenatally (2 infants in mediastinal septum and 4 infants in lung parenchyma), reported that BC grows slowly in the first months of life, but the growth is exponential even in the absence of complications. As a result, they recommend that complete resection should be done before the age of two to prevent infectious complications. Similarly, Fievet and colleagues studied the best time to conduct the operation in a series of 36 patients (11 children, 25 adults) with BC (17), and their findings show that surgery should be proposed between the 6th and 12th month of life, regardless of the existence of symptoms. For these reasons, we think that surgical resection should be performed as early as possible when the diagnosis of MBC was suspected, even in asymptomatic patients.
Although PLT cystectomy is the traditional surgical procedure for the treatment of MBCs, the application of VATS cystectomy has been increasing in infants and adults in the past decade. In our institute, VATS procedure for MBCs gradually became the most preferred approach with increased experience. The feasibility and validity of VATS in MBC have been discussed by numerous studies (1,6,14,18,28). However, most of these studies are case reports or case series, and appropriate comparative data to PLT have been sparse. Until now, only a small case series has evaluated the post-operative outcomes in children undergoing open verse thoracoscopic resection of bronchogenic cysts (29). Through review of the English literature, we found that our study was the first article evaluating the benefits of VATS for adults undergoing the excision of MBC by comparing between operation methods. Our data indicated that VATS procedure decrease the duration of chest drainage, which is in consistent with Tolg and colleagues’ study (29), and that VATS procedure did not increase the risk of incomplete resection, surgery-related complications, duration of intensive care unit stay, and postoperative complications. Moreover, our data also indicated that VATS procedure had a shorter operative time, a shorter postoperative hospital stay, less intra-operative blood loss, and less pleural drainage of the first three days after surgery. All of our results indicated that VATS cystectomy may be safe and feasible for the treatment of patients with MBC.
According to some reported studies, the conversion rate from VATS to open thoracotomy was 8–35%, and the main reason was major pleural adhesion (4,6,14). However, in our institution, VATS was initially performed in patients with lung cancer and some benign lung diseases, such as pneumothorax, tuberculous, and pulmonary aspergillosis. In these diseases, pleural adhesion or even atresia is not uncommon. We performed the first VATS operation to treat MBC in June 2006 and prior to this, we have accumulated a lot of experience. In our series, pleural atresia was observed in six patients in the VATS group. However, it does not seem to be an obstacle to perform a VATS excision. This result reflects the advantage of an accumulated learning experience in the process of VATS. In our series, only one patient (1/65, 1.54%) was converted to PLT due to vascular injury. Consistent with the result of Jung and colleagues (18), we also believe that pleural adhesions might not be the main reason for the conversion to thoracotomy of skilled hands.
In our study, one patient (1.54%) in VATS group suffered from postoperative bleeding compared with none in PLT group. This difference can be attributed to the learning curve because postoperative bleeding happened in PLT process as well. Therefore, it is reasonable to believe that some number of cases must be performed before surgeons can acquire sufficient proficiency.
Late recurrences have been described 0.67-25 years after surgery due to incomplete removal of all the cyst walls (30-32); thus, complete removal of the cyst should be attempted as much as possible (18). Nonetheless, the procedure may not always be smooth because it is not rare that cysts are severely adhered to nearby vital structures. In our study, 14 cysts were removed incompletely, leaving part of the cyst wall in situ. There were no differences in complete resection between the groups. We think that incomplete cyst excision is not attributable to the surgical approach but determined by the relationship between the cyst and the surrounding tissue. Electrocautery was performed in the remaining mucosal lining without recurrences for the patient with the longest follow-up response of 129 months. Similar to the results of other studies (33,34), we also think that the use of electrocautery may avoid cystic recurrences for incompletely resected patients. However, the results should be accepted carefully because of the small sample size, only conducting telephone follow-ups and relatively short follow-up period. In view of this, patients with incomplete resection should undergo long-term monitoring to prove the conclusion.
In conclusion, our findings show that both VATS and PLT are reliable approaches for the treatment of MBCs. The VATS approach is superior to PLT when considering perioperative results. This approach should be the preferred choice for the treatment of MBCs. However, this study was unavoidably limited by its single-institution, retrospective analysis, small sample size and the bias among different surgeons. Large prospective studies are suggested to confirm the conclusion.
Acknowledgements
Funding: This study was partially supported by the Key Science and Technology Program of Sichuan Province, China (2013SZ0005 and 2014SZ0148).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol of the study was approved by the Institutional Review Board (IRB) of West China Hospital (NO. 2016-168); informed consent was waived for this retrospective research.
References
- Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg 2000;69:1525-8. [Crossref] [PubMed]
- Granato F, Voltolini L, Ghiribelli C, et al. Surgery for bronchogenic cysts: always easy? Asian Cardiovasc Thorac Ann 2009;17:467-71. [Crossref] [PubMed]
- Granato F, Luzzi L, Voltolini L, et al. Video-assisted mediastinoscopic resection of two bronchogenic cysts: a novel approach. Interact Cardiovasc Thorac Surg 2010;11:335-6. [Crossref] [PubMed]
- De Giacomo T, Diso D, Anile M, et al. Thoracoscopic resection of mediastinal bronchogenic cysts in adults. Eur J Cardiothorac Surg 2009;36:357-9. [Crossref] [PubMed]
- Panchanatheeswaran K, Dutta R, Singh KI, et al. Eleven-year experience in thoracoscopic excision of bronchogenic cyst. Asian Cardiovasc Thorac Ann 2012;20:570-4. [Crossref] [PubMed]
- Weber T, Roth TC, Beshay M, et al. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg 2004;78:987-91. [Crossref] [PubMed]
- Liu W, Deslauriers J. Mediastinal divisions and compartments. Thorac Surg Clin 2011;21:183-90. viii. [Crossref] [PubMed]
- Langston C. New concepts in the pathology of congenital lung malformations. Semin Pediatr Surg 2003;12:17-37. [Crossref] [PubMed]
- Newman B. Congenital bronchopulmonary foregut malformations: concepts and controversies. Pediatr Radiol 2006;36:773-91. [Crossref] [PubMed]
- McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, et al. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology 2000;217:441-6. [Crossref] [PubMed]
- Croyle P, Estrera AS. Bronchogenic cyst and intralobar sequestration mimicking thoracic aortic aneurysm. South Med J 1982;75:1267-8. [Crossref] [PubMed]
- Black TL, Fernandes ET, Wrenn EL Jr, et al. Extralobar pulmonary sequestration and mediastinal bronchogenic cyst. J Pediatr Surg 1988;23:999-1001. [Crossref] [PubMed]
- Grewal RG, Yip CK. Intralobar pulmonary sequestration and mediastinal bronchogenic cyst. Thorax 1994;49:615-6. [Crossref] [PubMed]
- Muramatsu T, Shimamura M, Furuichi M, et al. Thoracoscopic resection of mediastinal bronchogenic cysts in adults. Asian J Surg 2011;34:11-4. [Crossref] [PubMed]
- Xu S, Liu B, Wang X, et al. Robotic thoracic surgery of the anterior superior mediastinal bronchogenic cyst. Ann Transl Med 2015;3:57. [PubMed]
- Maturu VN, Dhooria S, Agarwal R. Efficacy and Safety of Transbronchial Needle Aspiration in Diagnosis and Treatment of Mediastinal Bronchogenic Cysts: Systematic Review of Case Reports. J Bronchology Interv Pulmonol 2015;22:195-203. [Crossref] [PubMed]
- Fievet L, D'Journo XB, Guys JM, et al. Bronchogenic cyst: best time for surgery? Ann Thorac Surg 2012;94:1695-9. [Crossref] [PubMed]
- Jung HS, Kim DK, Lee GD, et al. Video-assisted thoracic surgery for bronchogenic cysts: is this the surgical approach of choice? Interact Cardiovasc Thorac Surg 2014;19:824-9. [Crossref] [PubMed]
- Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg 1992;53:1134-7. [Crossref] [PubMed]
- Patel SR, Meeker DP, Biscotti CV, et al. Presentation and management of bronchogenic cysts in the adult. Chest 1994;106:79-85. [Crossref] [PubMed]
- Miralles Lozano F, Gonzalez-Martínez B, Luna More S, et al. Carcinoma arising in a calcified bronchogenic cyst. Respiration 1981;42:135-7. [PubMed]
- Gotti G, Haid MM, Volteranni L, et al. Unusual malignancy in the wall of a mediastinal cyst. J Thorac Cardiovasc Surg 1993;106:1233-4. [PubMed]
- Endo C, Imai T, Nakagawa H, et al. Bronchioloalveolar carcinoma arising in a bronchogenic cyst. Ann Thorac Surg 2000;69:933-5. [Crossref] [PubMed]
- Maurin S, Hery G, Bourliere B, et al. Bronchogenic cyst: Clinical course from antenatal diagnosis to postnatal thoracoscopic resection. J Minim Access Surg 2013;9:25-8. [Crossref] [PubMed]
- Bush A. Prenatal presentation and postnatal management of congenital thoracic malformations. Early Hum Dev 2009;85:679-84. [Crossref] [PubMed]
- Michel JL, Revillon Y, Montupet P, et al. Thoracoscopic treatment of mediastinal cysts in children. J Pediatr Surg 1998;33:1745-8. [Crossref] [PubMed]
- Schier F, Waldschmidt J. Thoracoscopy in children. J Pediatr Surg 1996;31:1640-3. [Crossref] [PubMed]
- Jain P, Sanghvi B, Shah H, et al. Thoracoscopic excision of mediastinal cysts in children. J Minim Access Surg 2007;3:123-6. [Crossref] [PubMed]
- Tölg C, Abelin K, Laudenbach V, et al. Open vs thorascopic surgical management of bronchogenic cysts. Surg Endosc 2005;19:77-80. [Crossref] [PubMed]
- Read CA, Moront M, Carangelo R, et al. Recurrent bronchogenic cyst. An argument for complete surgical excision. Arch Surg 1991;126:1306-8. [Crossref] [PubMed]
- Gharagozloo F, Dausmann MJ, McReynolds SD, et al. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest 1995;108:880-3. [Crossref] [PubMed]
- Aslam MI, Abunasra H, Klimatsidas M, et al. Video-assisted mediastinoscopic drainage of a bronchogenic cyst presenting with cardiac dysfunction. Ann Thorac Surg 2009;88:1010-2. [Crossref] [PubMed]
- Lewis RJ, Caccavale RJ, Sisler GE. Imaged thoracoscopic surgery: a new thoracic technique for resection of mediastinal cysts. Ann Thorac Surg 1992;53:318-20. [Crossref] [PubMed]
- Kanemitsu Y, Nakayama H, Asamura H, et al. Clinical features and management of bronchogenic cysts: report of 17 cases. Surg Today 1999;29:1201-5. [Crossref] [PubMed]


