Treatment outcomes of patients with small cell lung cancer without prophylactic cranial irradiation
Introduction
Small cell lung cancer (SCLC) accounts for 15–20% of all lung cancer cases (1). As the number of elderly patients continues to increase, those aged ≥70 years with limited disease (LD) comprise approximately 50% of SCLC cases (2,3). SCLC advances rapidly and is likely to metastasize, whereas approximately 30% of cases are diagnosed as LD (4). Chemotherapy and radiotherapy are the mainstay treatments for SCLC, whereas platinum-containing chemotherapy is the primary regimen. For chemotherapy consisting of 4–6 courses of cisplatin or carboplatin with etoposide, the response rate is roughly 80% (5,6). Thoracic radiotherapy (TRT) administered in combination with anticancer agents is reported to increase the 3-year survival rate by 5% (7,8).
Although the response rate to radiotherapy and anticancer chemotherapy is relatively high, local recurrence and distant metastasis often occur within 2 years. Brain metastasis is the most common pattern of distant metastasis and is the only apparent site of relapse in 20–30% of patients with metastatic disease (9). Once brain metastasis occurs, prognosis is poor with a survival time of 4–6 months (10-12). Thus, it is important to design treatment regimens to prevent brain metastasis and maintain tumor control at the time of occurrence. In patients with SCLC, prophylactic cranial irradiation (PCI) is reported to improve prognosis (3). The effects of PCI have been investigated in many clinical studies, and this modality has been incorporated into standard treatment regimen (13,14). PCI is indicated for patients with LD who have achieved complete remission (CR) or near-CR with initial chemotherapy and radiotherapy. One study reported an incidence of brain metastasis and 3-year survival rate after PCI of 33.3% and 20.7%, respectively (3), indicating a significant improvement, as compared with those among patients who did not receive PCI. However, this report is rather dated; thus, these results may differ from those achieved with current medical treatments. Because of the advances in computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) devices, more rigorous cancer staging is now possible, thereby permitting more accurate selection of patients who will benefit from PCI.
Local recurrence and distant metastasis can be detected early by the use of a combination of CT, MRI, and evaluation of tumor markers, which allows for earlier therapeutic intervention. As second-line therapies have advanced and localized brain irradiation has become possible, treatment outcomes without the use of PCI are also expected to improve. In daily clinical practice, it is common to encounter patients who refuse PCI. Because PCI is often incorporated into standard treatment regimens for such cases, there is only a limited amount of well-documented data on the outcomes of treatment without PCI according to current medical standards. It will also be difficult to accumulate cases of patients treated without PCI in the future. Thus, the aim of this study was to examine overall survival (OS) and the incidence of brain metastasis, as well as to identify risk factors in patients with LD-SCLC who achieved CR or near-CR with initial therapy that did not include PCI.
Methods
Eligibility
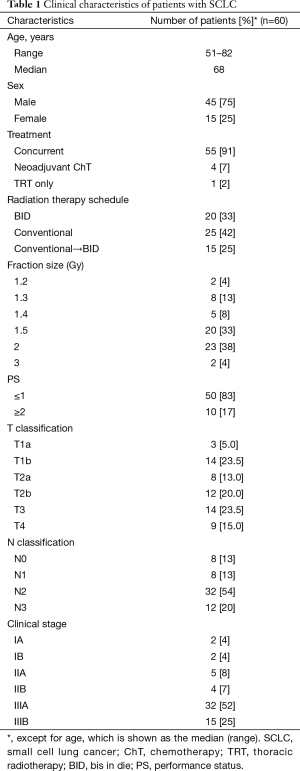
We retrospectively reviewed the records of 60 patients (45 men, 15 women; median age, 68 years; age range, 51–82 years) who were pathologically diagnosed with SCLC on bronchoscopy and treated between January 2001 and May 2014. Of these, we retrospectively analyzed the clinical data of those with LD who achieved CR or near-CR with initial chemotherapy and radiotherapy but did not receive PCI. Patients with concomitant cancer at other sites and those being treated for previous cancer were excluded. Moreover, patients with serious lung or heart diseases who required continuous oxygen administration were also excluded.
All patients underwent a standardized evaluation that included blood testing, tumor marker analysis, and contrast-enhanced whole-body CT (plus PET for recently treated patients). Contrast-enhanced head MRI or CT was performed to confirm the absence of brain metastasis, whereas bone scintigraphy was performed when bone metastasis was suspected. Staging was determined in accordance with the Union for International Cancer Control guidelines, seventh edition. Staging, therapeutic effects, and the occurrence of brain metastasis were determined by respiratory physicians, diagnostic radiologists, and radiation oncologists. LD included patients with lesions confined to the ipsilateral hemithorax or the regional and supraclavicular lymph nodes. Patients with metastasis to other sites were defined as having extensive disease (ED) and subsequently excluded from analysis. Therapeutic effects were determined using contrast-enhanced CT. However, when they were difficult to evaluate, for example, in the presence of radiation pneumonitis, treatment was considered as effective by the confirmation of the normalization of levels of neuron-specific enolase and pro-gastrin-releasing peptide, which are useful tumor markers, or the absence of accumulation on PET. Survival time was defined as the time from the completion of radiotherapy to death. The time of brain metastasis occurrence was defined as the time when brain metastasis was confirmed on contrast-enhanced CT or MRI after radiotherapy.
Treatment
Radiation treatment planning was performed using a CT simulator (long scan time, 3 s). Regarding primary lesions, because a long scan time was used, the gross tumor volume (GTV) was not specified, but areas visible on CT were used as the clinical target volume (CTV). Regarding lymph node metastasis, CTV was defined as GTV with a margin of 5 mm. On elective nodal irradiation, lymph node areas were also included in CTV. The planning target volume (PTV) was defined as CTV with a margin of 5–7 mm. PTV with a leaf margin of 5 mm was used as the radiation field. When anticancer chemotherapy preceded radiotherapy, PTV was determined with CTV based on images taken after achieving a reduction in tumor size. Two or more multiple-field irradiation techniques were used and radiation doses were calculated with heterogeneity correction. Irradiation was performed with 6–10 MV photon beams (MVX). The dose fractionation schedules were 1.2, 1.3, 1.4, 1.5, 2, and 3 Gy, and the total radiation dose was 36–60 Gy (median, 50 Gy). While twice-daily or bis in die (BID) irradiation was selected as the basic schedule, conventional irradiation at 2 Gy/fraction was administered to patients who did not concurrently receive anticancer chemotherapy and those with concerns about adverse reactions. Some patients received irradiation once a day at the start of radiotherapy, followed by BID irradiation to prevent regrowth only after temporary discontinuation of radiotherapy due to adverse reactions. For these patients, the single dose used in BID irradiation was 1.2, 1.3, 1.4, or 1.5 Gy, adjusted according to the general condition of each patient. For patients who developed brain metastasis, whole-brain irradiation was performed using a CT simulator. Patients with brain metastasis were irradiated on a linear accelerator at 10 MVX once daily for 5 days per week. Since the orbits were included in the radiation field, for patients receiving whole-brain irradiation, who were suspected of having cancer dissemination, eye shields were utilized, the lower margin of the radiation field was set at the C2 vertebra, and two opposing lateral fields were used.
Chemotherapy was concurrently started with radiotherapy. When radiotherapy was started within 1 month after the start of anticancer chemotherapy, treatment was considered concurrent. As the basic regimen, cisplatin (80 mg/m2 on day 1) and etoposide (100 mg/m2 on days 1, 2, and 3) were administered every 4 weeks for four courses. Depending on renal function and age, carboplatin was also administered to some patients. The doses of the anticancer agents were adjusted according to blood cell counts and renal function measurements. When the leukocyte or platelet count was decreased by the administration of the anticancer agents, they were discontinued and granulocyte colony-stimulating factors were administered. After the count improved, chemotherapy was resumed. When the serum creatinine level was elevated during treatment, the doses of the anticancer agents were reduced in the subsequent course.
After treatment completion, blood tests and chest radiography were performed approximately every 3 months at the outpatient unit. Whole-body CT scans were performed every 6 months or when the tumor marker levels were elevated. While some patients underwent brain MRI every 6 months, others underwent brain MRI when they developed symptoms, such as headache or lightheadedness. When brain metastasis occurred, whole-brain irradiation was immediately performed. Moreover, when local recurrence or distant metastasis occurred, second-line therapy with amrubicin (AMR) was started.
Statistical analysis
The OS time was defined as the time from radiation therapy to death or until the end of the follow-up period in May 2014. Time to progression to the brain was defined as the time from radiotherapy to confirmation of brain metastases by imaging diagnostics. Survival was calculated using the Kaplan-Meier method and differences were confirmed with the log-rank test. Independent variables that were found to be statistically significant on univariate analysis were tested by multivariate Cox regression analysis. A probability (P) value <0.05 was considered statistically significant. All calculations were conducted using SPSS 15.0J statistical software (IBM-SSPS, Inc., Chicago, IL, USA).
Results
Patient and treatment characteristics
Baseline patient characteristics are listed in Table 1. Between January 2005 and May 2014, the data of 60 patients (45 men, 15 women) were eligible for analysis. The median patient age was 68 years (51–82 years). The median follow-up period was 23 months (2–108 months). Concurrent administration of cisplatin or carboplatin, etoposide, and radiotherapy was selected as the basic procedure and administered in 55 patients. There were four patients in whom chemotherapy preceded radiotherapy due to large tumors, whereas one patient received radiotherapy only because anticancer agents could not be administered because of cardiac complications and renal impairment. Although BID irradiation at a single dose of 1.5 Gy was selected as the basic schedule, only 20 patients received it. Fifteen patients started with conventional irradiation at 2 Gy/fraction and received BID irradiation only in the latter half of the treatment period. The BID irradiation dose in the latter half was 1.2 Gy/fraction in 2 patients, 1.3 Gy/fraction in 8, and 1.4 Gy/fraction in 5. The doses of conventional irradiation were 2 Gy/fraction in 23 patients and 3 Gy/fraction in 2. The total radiation dose was 36–60 Gy. For brain metastasis, whole-brain irradiation was performed at a single dose of 2.5 or 3 Gy, resulting in a total dose of 30 or 37.5 Gy. All patients achieved CR or near CR after initial therapy. However, all patients refused PCI or could not tolerate PCI because of adverse reactions.
Full table
Survival
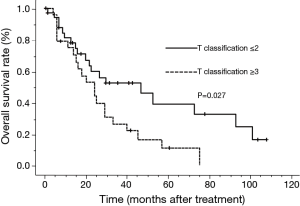
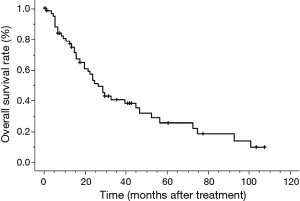
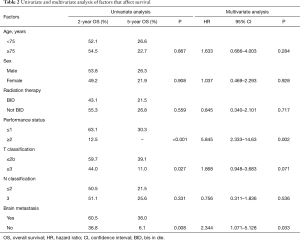
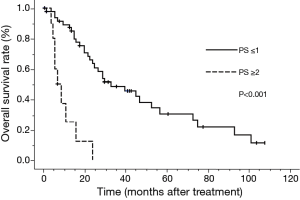
The median survival time (MST) for this patient population was 25 months. The OS rates at 2 and 5 years were 52.6% and 25.3%, respectively (Figure 1). The clinical factors evaluated to determine their prognostic value for OS are summarized in Table 2. Univariate analysis revealed that the development of brain metastasis, performance status (PS), and T-stage were significant factors correlated with survival rate. Survival was significantly longer among patients who did not develop brain metastasis than in those who did, with 2- and 5-year OS rates of 60.5% and 36.0% vs. 36.8% and 6.1%, respectively (P=0.008; Figure 2). In addition, survival was significantly longer among patients with PS ≤1 than for those with PS ≥2, with 2- and 5-year OS of 63.1% and 30.3% vs. 12.5% and no calculated value available, respectively (P<0.001; Figure 3). Survival was significantly longer among patients with T-stage ≤2 than those with T-stage ≥3, with 2- and 5-year OS of 59.7% and 39.1% vs. 44.0% and 11.0%, respectively (P=0.027; Figure 4). Multivariate analysis identified only PS [hazard ratio (HR), 5.845, 95% confidence interval (CI), 2.333–14.63, P=0.002] and brain metastasis as independent prognostic variables (HR, 2.344, 95% CI, 1.071–5.128, P=0.033).
Full table
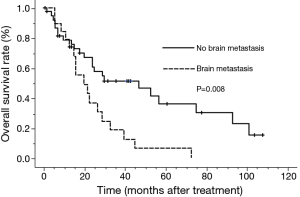
Overall survival in patients of SCLC with CR after ChT and TRT. The median survival time was 25 months. SCLC, small cell lung cancer; CR, complete remission; ChT, chemotherapy; TRT, thoracic radiotherapy.
Patterns of failure
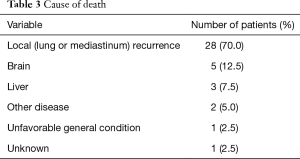
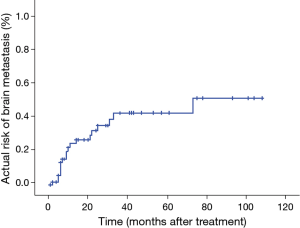
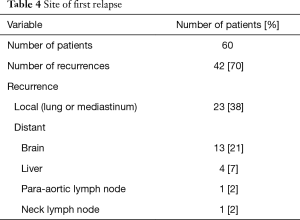
Forty patients died during the follow-up period. The causes of death were recurrence in the lungs in 28 patients, brain metastasis in 5, liver metastasis in 3, other diseases in 2, unfavorable general condition in 1, and unknown in 1 (Table 3). Nineteen patients (19/60, 32%) developed brain metastasis. The time for development of brain metastasis was 2–73 months, with a median time of 10 months, which was calculated from TRT. The actuarial risks of brain metastasis for all patients at 1 and 3 years were 23.8% and 41.3%, respectively (Figure 5). Brain metastasis occurred as the first relapse in 13 patients (Table 4). There were six patients with preceding pulmonary lesions. Of the 19 patients who developed brain metastasis, 2 had N0 tumors and 1 had N1 tumors. The other patients had N2 or N3 tumors. Although the incidence of brain metastasis was higher among patients with N2 or greater tumors, there was no apparent statistically significant difference. There were no significant correlations among the other factors and the incidence of brain metastasis. Of the 19 patients with brain metastasis, 14 received whole-brain irradiation, whereas the other 5 did not. The median time from the diagnosis of brain metastasis to death was 6 months (1–22 months).
Full table
Full table
Discussion
Chemotherapy combined with TRT is the standard treatment for the management of LD (5). For patients achieving CR or near-CR after initial therapy, prompt PCI is widely known as the standard treatment (13,14). Regarding doses of whole-brain irradiation, even increased radiation doses do not improve outcomes, and the standard dose is 25 Gy in ten daily fractions of 2.5 Gy each (15). Aupérin et al. (3) performed a meta-analysis of 987 patients, who achieved CR, selected among patients enrolled in seven clinical studies conducted between 1965 and 1995, and reported that when only patients in CR (including those in CR as determined by plain chest radiography) were analyzed, PCI significantly reduced the 3-year recurrence rate of brain metastasis from 58.6% to 33.3% and significantly improved the 3-year survival rate for patients with LD and ED from 15.3% to 20.7%.
To the best of our knowledge, no report has indicated that patients with LD in CR can be treated without PCI. Bree et al. reported that although the effects of PCI were not apparent in patients ≥80 years old, PCI significantly improved outcomes in other elderly patients (16). Since approximately 15 years have elapsed since the usefulness of PCI was first reported, no doubt of its effects remains. However, there are cases in which PCI cannot be performed due to varied reasons, including concerns about adverse reactions to PCI and patient request to forego the procedure. A previous report by Patel et al. indicated that PCI was administered to only 8% of patients with LD (17). At present, although the proportion of patients receiving PCI is presumably increasing, it is expected that some patients will not receive PCI for various reasons in actual clinical practice.
Because PCI is incorporated into the standard treatment regimen, there are relatively few reports on treatment outcomes in patients who do not receive PCI despite CR being achieved with initial therapy. Thus, this study aimed to examine the outcomes of treatment without PCI and the incidence of brain metastasis in patients with LD who achieved CR. Our results were better than previously reported outcomes of chemoradiotherapy and comparable to those of complete surgical resection. Zhu et al. assessed the outcomes of patients with completely resected pathological stage II or III SCLC who did not receive PCI and reported 2-year survival rates of 78.6% among patients with stage II disease and 42.6% for those with stage III disease, with 5-year survival rates of 61.7% and 26.6%, respectively (18). Thus, these results should be considered reasonably favorable. Moreover, Tsuchiya et al. reported that after complete resection in patients with clinical stage I to IIIA SCLC, who were also treated with cisplatin and etoposide, the 5-year survival rates were 66%, 56%, and 13% for clinical stage I, II, and IIIA disease, respectively (19). These results appear highly accurate in terms of staging based on postoperative findings. Despite the differences in pathological and clinical stages, the surgical outcomes in these reports and the results of the present study are assumed to be comparable given that surgically resected SCLC is considered as a CR in SCLC.
The results of the present study revealed that the outcomes of treatment without PCI were improved, as compared with previously reported results. Although there has been no change in the strategy of concurrent administration of chemotherapy, mainly with cisplatin combined with etoposide and radiotherapy, the improved outcomes are attributable to the stricter definition of CR based on contrast-enhanced CT and tumor markers than the previous definitions, improved radiation techniques, and post-treatment follow-up with assessment using CT and tumor markers, which allows for a prompt transition to second-line therapy at the time of recurrence. Regarding second-line therapy, a subgroup analysis of a phase III study on nogitecan (NGT) and AMR for recurrent SCLC revealed that MST was 6.2 months among patients receiving AMR and 5.7 months for those receiving NGT, showing a significantly prolonged survival time in those receiving AMR (20). At our institution, treatment with AMR and improved systemic management also appeared to have contributed to the improved outcomes. However, it should be considered that the outcomes in the present study may have been affected by the patient characteristics of lower median age than in other reports and the relatively larger number of patients with good PS.
Arriagada et al. reported that the incidence of brain metastasis at the first relapse site at 5 years was 37% in a group of patients not receiving PCI and 20% in those receiving PCI (21). A recent retrospective report also indicated a 25% incidence in the development of brain metastasis after PCI (22,23). Moreover, when PCI was not performed for patients with completely surgically resected SCLC, the actuarial risk of developing brain metastasis was reportedly 13.8% at 1 year and 23.0% at 3 years (18). In contrast, the incidence of brain metastasis in the present study was higher. Because these patients already had multiple recurrence sites at the time of brain metastasis detection, stereotactic irradiation was difficult to perform. In addition, since there are reports that central nervous system metastasis occurs in 80% of patients, including autopsy cases, within 2 years after treatment, it is assumed that the incidence of microscopic metastasis undetectable by imaging studies increases over time (3,24). Thus, even though brain metastasis can be detected early by close follow-up, we speculate that relatively few patients are suitable for treatment with stereotactic irradiation. The most common site of first relapse after CR was the local site, followed by the brain. Significant differences in survival were observed between patients with and without brain metastasis. Once brain metastasis occurred, the median MST was as short as 6 months (1–22 months). On the basis of these data, the prevention of brain metastasis by PCI is assumed to contribute to improved prognosis.
Symptoms of acute toxicity of PCI consisted of headache, fatigue, nausea, and vomiting, which were usually manageable. However, symptoms of long-term toxicity, such as memory loss, intellectual impairment, ataxia, and seizure, are of concern. Nonetheless, it has been reported that baseline neuropsychological testing showed no abnormality in only 41% of patients with SCLC. No significant difference was observed between those receiving or not receiving PCI (25). Patients with SCLC exhibit cognitive deficits, as compared with the normal population before PCI, and these abnormalities may even be present at the time of diagnosis. These findings are now widely acknowledged (26). Although smoking, paraneoplastic syndrome, and the effects of chemotherapy are considered the causes of these abnormalities, some authors deny the impact of the enhanced toxicity of PCI. On the basis of these data, we believe that PCI should not be omitted because of the risk of adverse reactions. However, the follow-up period was as short as 1–2 years and late-stage neurotoxicity in long-term surviving patients has not yet been elucidated.
Our study has limitations, including its relatively small sample size compare with past study. Because PCI is already known as a component of standard treatment, the number of patients that did not receive was limited. Although a clinical study is also difficult to conduct, at our institution, we perform PCI for patients in CR as part of the standard treatment. However, because we considered patient age, general condition, and wishes in previous cases, there are few well-documented cases in which PCI was not performed. Thus, we are unable to compare the outcomes of treatment with and without PCI in our cohort, and the outcomes for patients not receiving PCI had to be compared with the results described in past reports. Second, TRT schedule and variety of fractionations can be a bias in interpreting data of survival. Furthermore, because the number of patients who developed brain metastasis was small, we were unable to elucidate any risk factors of developing brain metastasis.
The results of our study demonstrated that the outcomes of treatment without PCI were improved, as compared with those of previously published data. However, because a significant difference in OS was observed between patients with and without brain metastasis, PCI should not be routinely omitted. The findings presented here may be used as reference data when PCI cannot be performed for various reasons.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in the studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We informed merit and demerit of the PCI and obtained consent of all patients.
References
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Lally BE, Geiger AM, Urbanic JJ, et al. Trends in the outcomes for patients with limited stage small cell lung cancer: An analysis of the Surveillance, Epidemiology, and End Results database. Lung Cancer 2009;64:226-31. [Crossref] [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin 2006;56:106-30. [Crossref] [PubMed]
- Semrau S, Bier A, Thierbach U, et al. 6-year experience of concurrent radiochemotherapy with vinorelbine plus a platinum compound in multimorbid or aged patients with inoperable non-small cell lung cancer. Strahlenther Onkol 2007;183:30-5. [Crossref] [PubMed]
- Mascaux C, Paesmans M, Berghmans T, et al. A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 2000;30:23-36. [Crossref] [PubMed]
- Arriagada R, Pignon JP, Ihde DC, et al. Effect of thoracic radiotherapy on mortality in limited small cell lung cancer. A meta-analysis of 13 randomized trials among 2,140 patients. Anticancer Res 1994;14:333-5. [PubMed]
- Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 1992;10:890-5. [PubMed]
- Viani GA, Boin AC, Ikeda VY, et al. Thirty years of prophylactic cranial irradiation in patients with small cell lung cancer: a meta-analysis of randomized clinical trials. J Bras Pneumol 2012;38:372-81. [Crossref] [PubMed]
- Socha J, Kępka L. Prophylactic cranial irradiation for small-cell lung cancer: how, when and for whom? Expert Rev Anticancer Ther 2012;12:505-17. [Crossref] [PubMed]
- Seute T, Leffers P, ten Velde GP, et al. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer 2004;100:801-6. [Crossref] [PubMed]
- Komaki R, Cox JD, Whitson W. Risk of brain metastasis from small cell carcinoma of the lung related to length of survival and prophylactic irradiation. Cancer Treat Rep 1981;65:811-4. [PubMed]
- Kotalik J, Yu E, Markman BR, et al. Practice guideline on prophylactic cranial irradiation in small-cell lung cancer. Int J Radiat Oncol Biol Phys 2001;50:309-16. [Crossref] [PubMed]
- Mehta MP. Models support prophylactic cranial irradiation. J Clin Oncol 2006;24:3524-6. [Crossref] [PubMed]
- Le Péchoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467-74. [Crossref] [PubMed]
- Eaton BR, Kim S, Marcus DM, et al. Effect of prophylactic cranial irradiation on survival in elderly patients with limited-stage small cell lung cancer. Cancer 2013;119:3753-60. [Crossref] [PubMed]
- Patel S, Macdonald OK, Suntharalingam M. Evaluation of the use of prophylactic cranial irradiation in small cell lung cancer. Cancer 2009;115:842-50. [Crossref] [PubMed]
- Zhu H, Bi Y, Han A, et al. Risk factors for brain metastases in completely resected small cell lung cancer: a retrospective study to identify patients most likely to benefit from prophylactic cranial irradiation. Radiat Oncol 2014;9:216. [Crossref] [PubMed]
- Tsuchiya R, Suzuki K, Ichinose Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). J Thorac Cardiovasc Surg 2005;129:977-83. [Crossref] [PubMed]
- Giaccone G, Ferrati P, Donadio M, et al. Reinduction chemotherapy in small cell lung cancer. Eur J Cancer Clin Oncol 1987;23:1697-9. [Crossref] [PubMed]
- Arriagada R, Le Chevalier T, Rivière A, et al. Patterns of failure after prophylactic cranial irradiation in small-cell lung cancer: analysis of 505 randomized patients. Ann Oncol 2002;13:748-54. [Crossref] [PubMed]
- Alshehadat S, Sahmoun AE. Prophylactic cranial irradiation in limited-stage small-cell lung cancer: a retrospective analysis. Clin Adv Hematol Oncol 2004;2:397-400. [PubMed]
- Stanic K, Kovac V. Prophylactic cranial irradiation in patients with small-cell lung cancer: the experience at the Institute of Oncology Ljubljana. Radiol Oncol 2010;44:180-6. [Crossref] [PubMed]
- Cooper S, Spiro SG. Small cell lung cancer: treatment review. Respirology 2006;11:241-8. [Crossref] [PubMed]
- Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst 1995;87:183-90. [Crossref] [PubMed]
- Wright J, Wolfson A. Prophylactic cranial irradiation: do benefits outweigh neurocognitive impact? Curr Probl Cancer 2012;36:106-16. [Crossref] [PubMed]