Prognostic value of preoperative serum lactate dehydrogenase in thymic carcinoma
Introduction
Thymic epithelial tumors (TETs), which include thymoma and thymic carcinoma, are most common tumors in the anterior mediastinum (1). Thymic carcinoma, which accounts for 10% of TETs approximately, can be distinguished from thymoma by its clinicopathological and biological characteristics. Unlike thymoma, thymic carcinoma is aggressive and has a generally poor prognosis, with a 5-year survival rate of approximately 40% (2-4). Complete resection is the first line treatment of operable thymic carcinomas; radiotherapy and chemotherapy appear to benefit patients with inoperable or incompletely resected tumors (5-7).
Recently, metabolic reprogramming has been recognized as a hallmark of cancer (8). In the presence of oxygen, most normal cells metabolize glucose to carbon dioxide and water by oxidation of glycolytic pyruvate via the tricarboxylic acid (TCA) cycle, which occurs in the mitochondrial matrix. The reaction produces the reduced form of nicotinamide-adenine dinucleotide (NADH), which participates in mitochondrial oxidative phosphorylation (OXPHOS) to maximize adenosine triphosphate (ATP) production. Cancer cells, on the other hand, preferentially metabolize pyruvate via the glycolysis pathway regardless of available oxygen, which results in production of relatively large amounts of lactate (9) and creates an acidic microenvironment that promotes tumor growth and metastasis (10).
This abnormal metabolic preference for aerobic glycolysis is known as the ‘Warburg effect’ (11), and the last step in the pathway, conversion of pyruvate to lactate, is reversibly catalyzed by lactate dehydrogenase (LDH). Increased serum LDH levels detected before treatment have been shown to indicate poor prognosis in a number of solid tumors, including breast (12), colorectal (13), gastric (14), and renal cell cancer (15), hepatocellular carcinoma (HCC) (16) and nasopharyngeal carcinoma (NPC) (17). Serum LDH has also been proposed as a biomarker of distant metastasis in the TNM staging of melanoma by the European and American Joint Committee on Cancer (AJCC) (18). In this study, we investigated the possible correlation of serum LDH level with prognosis of thymic carcinoma.
Methods
Patients
A total of 106 consecutive patients with pathologically confirmed thymic carcinoma were treated surgically in the department of Thoracic Surgery of the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, between January 2005 and December 2015. All were considered for inclusion. Eleven patients who received radiotherapy and/or chemotherapy before surgery or had coexistent malignancies or other comorbidities, such as inflammation, anemia, severe lung or liver disease, all of which might influence serum LDH concentration, were excluded. The remaining 95 patients were included in the analysis. Complete resection was possible in 76 patients and subtotal resection or debulking surgery was carried out in 19. Complete resection included thymectomy, dissection of enlarged regional lymph nodes and mediastinal fat tissue, and resection of adjacent invaded tissues. Incomplete resection included subtotal removal and debulking in patients with aggressive lesions that could not be completely removed. Patients with complete resection, who were considered to have a high risk of recurrence based on operative findings, and all those with incomplete resection, were offered postoperative radiotherapy and/or chemotherapy in our department. The study followed the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Informed consent was not required for this retrospective study.
Serum LDH
Serum LDH levels were determined within 1 week of surgery and assayed spectrophotometrically by a standard enzyme-based method (Roche Holding AG, Basel, Switzerland) in the clinical laboratory of our hospital following the standards of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). The normal reference range of serum LDH was 135–225 U/L, and the coefficient of variance of the LDH assay was <6.0%. Patients were stratified into two groups by LDH concentration; >225 U/L, the ULN, or ≤225 U/L.
Data collection
Patient demographic and clinicopathological characteristics were obtained by review of their medical records. Age, sex, tumor size, serum LDH levels, histologic classification, staging information, surgical approach, and adjuvant therapy were collected. All cases were staged according to the Masaoka system, and histologic classification of thymic carcinoma was based on the World Health Organization (WHO) histologic criteria (19). In patients with complete resection, staging and histologic classification were determined by postoperative histopathological analysis. In patients with incomplete resection, intraoperative and pathological findings, description of tumor characteristics, lymph nodes, and distant metastasis mainly depended on systemic physical examination, chest computed tomography (CT) scans, cervical and abdominal ultrasound, and radionuclide whole bone scans, and/or position emission tomography-computed tomography (PET-CT) before surgery. Overall survival (OS) was defined as the interval between the date of surgery and the date of death from any cause or the last follow-up. Progression-free survival (PFS) was defined as the interval between the date of surgery and regional disease progression (mainly for cases with incomplete resection), the first recurrence or metastasis, or the last follow-up.
Statistical analysis
Values of continuous variables were expressed as means ± standard deviation (SD) or medians and range. Categorical variables were reported as numbers and percentages. Patients who were alive at the end of follow-up were censored from the analysis of OS. The Kaplan-Meier method was used to calculate the 1-, 3-, and 5-year OS and 1-, 3-, and 5-year PFS. Cox proportional regression analysis was used to determine the significance of associations between each variable, OS, and PFS. Variables found to be associated with survival in the univariate analysis were further tested in a multivariate model stepwise when α=0.05. The association between each continuous variable and LDH level, i.e., ≤225 or >225 U/L, was evaluated using the t-test. The association between each categorical variable and LDH level was evaluated using the chi-square test. Kaplan-Meier plots were calculated to estimate survival stratified by significant clinical variables with differences tested for significance by the log-rank test. All tests were two-sided, and P values <0.05 were considered significant. All data were analyzed by SPSS 19.0 (IBM Corp. Armonk, NY, USA).
Results
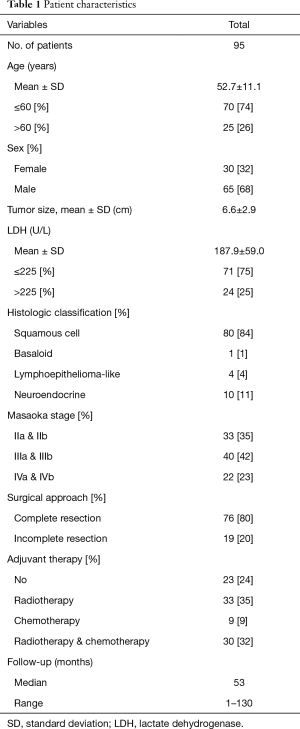
Patient characteristics
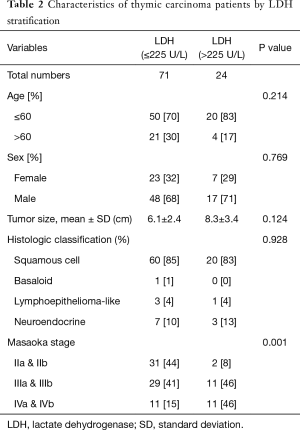
The demographic and clinical characteristics of the 95 enrolled patients are shown in Table 1. The Masaoka stage of patients with high LDH levels >225 U/L was more advanced than that of patients with low LDH levels ≤225 U/L (P=0.001). However, serum LDH levels were not significantly associated with age (P=0.214), sex (P=0.769), tumor size (P=0.124) and histologic classification (P=0.928) (Table 2).
Full table
Full table
PFS
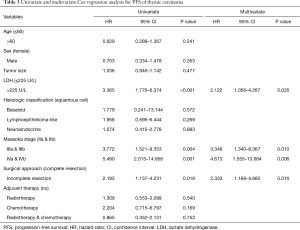
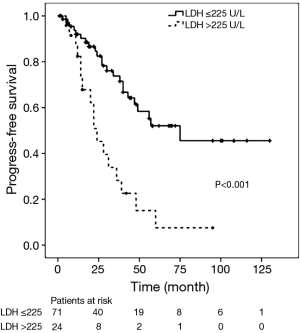
During the follow-up period, the 1-, 3-, and 5-year PFS rates were 76%, 51% and 38% respectively. Disease progression (regional progression, recurrence, or metastasis) was observed in 40 patients (42%). In the Cox univariate regression analysis, LDH level (HR =3.365; 95% CI: 1.776–6.374; P<0.001), Masaoka stages IIIa and IIIb (HR =3.772; 95% CI: 1.521–9.353; P=0.004, IVa and IVb (HR =5.490; 95% CI: 2.015–14.958; P=0.001), and surgical approach (HR =2.193; 95% CI: 1.137–4.231; P=0.019) were significantly associated with PFS. Age (P=0.241), sex (P=0.353), tumor size (P=0.477), histological classification (P>0.05) and adjuvant therapy (P>0.05) were not significantly associated with PFS. Cox multivariate regression analysis found that LDH level (HR =2.122; 95% CI: 1.056–4.267; P=0.035), Masaoka stages IIIa and IIIb (HR =3.348; 95% CI: 1.340–8.367; P=0.010), IVa and IVb (HR =4.613; 95% CI: 1.555–13.684; P=0.006), and surgical approach (HR =2.333; 95% CI: 1.168–4.660; P=0.016) remained as independent prognostic factors (Table 3). Kaplan-Meier analysis found high serum LDH level was associated with a significantly increased risk of disease progression, recurrence or metastasis (P<0.001, log-rank test) (Figure 1).
Full table
OS
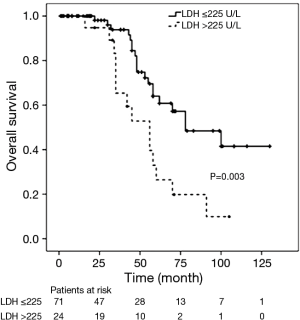
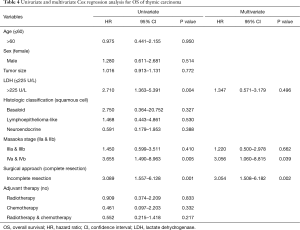
There were 34 deaths (36%) during follow-up, and Kaplan-Meier analysis indicated 1-, 3-, and 5-year OS rates of 97%, 75% and 46% respectively. Of the 34 deaths, 32 (94%) patients died from tumor progression, 1 (3%) patient died from acute cerebral infarction and 1 (3%) case died from unknown cause. In the Cox univariate regression model, serum LDH level (HR =2.710; 95% CI: 1.363–5.391; P=0.004), Masaoka stages IVa and IVb (HR =3.655; 95% CI: 1.490–8.963; P=0.005), and surgical approach (HR =3.089; 95% CI: 1.5597–6.128; P=0.001) were significantly associated with OS. However, age (P=0.950), sex (P=0.514), tumor size (P=0.772), histologic classification (P>0.05) and adjuvant therapy (P>0.05) were not significantly associated with OS. In the Cox multivariate model, advanced Masaoka stages IVa and IVb (HR =3.056; 95% CI: 1.060–8.815; P=0.039) and incomplete resection (HR =3.054; 95% CI: 1.508–6.182; P=0.002) indicated of increased risk of death, i.e., indicated poor prognosis, while LDH level (P=0.496) was not independently associated with OS (Table 4). Kaplan-Meier analysis and log-rank tests found that patients with serum LDH >225 U/L had a significantly shorter OS than other thymic carcinoma patients (P=0.003, Figure 2).
Full table
Discussion
In this study of serum LDH level and prognosis of thymic carcinoma, we found that increased preoperative serum LDH levels were significantly associated with the Masaoka stage, disease progression, and poor survival. Moreover, when patients were stratified by serum LDH level found that the subgroup with an LDH concentration above the 225 U/L ULN had shorter OS and PFS than the subgroup with a an LDH level at or below the 225 U/L threshold. Furthermore, we found that high serum LDH was significantly associated with an advanced Masaoka stage. Preoperative serum LDH level was an independent prognostic factor of PFS in patients with thymic carcinoma. Although it was not an independent predictor of death, a high preoperative LDH level was associated with decreased OS. Consequently, the results support use of preoperative serum LDH level as a prognostic marker for patients with thymic carcinoma. In addition, both Masaoka stage and complete resection were independent prognostic indicators of OS and PFS in these thymic carcinoma patients, which is consistent with previous reports (20,21). Patients with thymic carcinoma and complete resection are known to have better survival than those with incomplete resection (20). The 5-year OS and the 5-year PFS of thymic carcinoma patients in our hospital were comparable to those reported in other retrospective studies (2-4,22,23) (46% vs. an average of 40% and 38% vs. an average 42%, respectively).
In the 1920s, Otto Warburg observed that lactate was produced almost exclusively during hypoxia and, was not reduced in cancer tissues by the presence of sufficient oxygen, and production even exceeded that of normal tissue. This remarkable phenomenon was termed the “Warburg effect” or “aerobic glycolysis” by Racker and Spector (24). Nevertheless, in succeeding decades, cancer has come to be considered as exclusively driven by the activation of proto-oncogenes and functional deficiency of tumor-suppressor genes (25). Warburg’s findings and his hypothesis of carcinogenesis were ignored by most cancer researchers until Hanahan and Weinberg demonstrated in 2011 that reprogramming of energy metabolism was an important event in cancer (8,26). The metabolic program of tumor cells continuously adapts to deal with the challenges of harsh environments (27). Hypoxia gradients, which are characteristic of solid tumors (28), induce the constitutive expression of hypoxia-inducible factor (HIF)-1, an important transcriptional factor. HIF-1 is responsible for a nutrient-driven genomic response that allows cancer cells to adapt to hypoxia by increasing glycolysis (29). It just allows cancer cells to metabolize glucose through the glycolysis pathway instead of aerobic respiration. The continued consumption of glucose gives rise to a glycolytic flux that is adequate to produce the ATP needed for tumor growth. However, pyruvate dehydrogenase (PDH), which transfers pyruvate to the TCA cycle, cannot meet the requirements of high glycolytic flux. Consequently, in tumor cells the pyruvate generated by glycolysis is converted into lactate and protons by LDH (30). Excess lactate and protons produced by glycolysis are secreted from the cytoplasm and produce an acidic microenvironment that promotes cancer cell proliferation, neovascularization, and can even trigger apoptosis of adjacent normal cells, all of which facilitate tumor invasion and metastasis (31-33). The molecular events underlying the Warburg effect are not clearly understood. However, it has been proposed that mitochondrial uncoupling, rather than irreversible OXPHOS defects, drives the metabolic reprogramming of tumor cells (34).
LDH is an enzyme involved in anaerobic glycolysis, and is regulated by the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (mTOR)-containing complex 1 (PI3K/Akt/mTORC1) signal transduction pathway, tumor hypoxia, or nutrient deprivation (35). It is a cytoplasmic enzyme that reversibly catalyzes the transformation of pyruvate to lactate and protons. It is highly expressed and activated in solid tumors that have increased rates of glycolysis and lactate production. The acidity generated by lactate and protons stimulates epithelial-mesenchymal transition (EMT), invasiveness, and metastatic dissemination of cancer cells, which are correlated with resistance to therapy and poor clinical prognosis (36). This might explain the decreased survival of patients with high serum LDH levels. LDH has two subunits, LDH-A (also known as the M subunit) and LDH-B (also known as the H subunit) that are encoded by separate genes (A and B). LDH-B is the major form of LDH in serum, while LDH-A, which is present in smaller amounts, is closely correlated with cancer metabolism (37,38). The LDH-A and LDH-B polypeptides form tetramers to generate five LDH isoenzymes, LDH-1 to LDH-5, which have tissue specificity. LDH-1 includes four LDH-B subunits, and LDH-5 includes four LDH-A subunits. LDH-A primarily catalyzes conversion of pyruvate to lactate in hypoxic microenvironments; LDH-B kinetically favors the reverse conversion, i.e., lactate to pyruvate. Increase in the number of LDH-A subunits (LDH-5) in a tetramer favors the conversion of pyruvate to lactate; increase of LDH-B subunits (LDH-1) favors the conversion of pyruvate to acetyl-CoA that enters the TCA cycle (39,40). A recent meta-analysis of 12 studies concluded that LDH-5 overexpression is associated with poor survival in patients with solid tumors, including head and neck squamous cell cancer, colorectal adenocarcinoma, renal clear cell carcinoma, oral squamous cell carcinoma, non-small cell lung cancer (NSCLC), melanoma, gastric cancer, and endometrial cancer (41).
The LDH-1 isoenzyme has been proposed as an important marker of germ cell tumors, particularly ovarian and testicular tumors (42). However, LDH-A and LDH-B have often been studied individually, which may impede a deeper understanding of their roles in cancer metabolism. Recent evidence indicates that the ratio of LDH-B to LDH-A might be a biomarker of tumor aggressiveness, particularly for triple-negative breast cancers (TNBCs) (43), which highlights the importance of interaction between LDH-A and LDH-B in cancer progression.
The prognostic value of serum LDH has been confirmed in both hematological malignancies and solid tumors. High serum LDH level predicts poor survival in diffuse large B-cell lymphoma (DBCL) and is one of the five risk factors included in the International Prognostic Index (IPI) for patients with DBCL (44,45). A recent meta-analysis found that high serum LDH levels were associated with significant HRs of 1.7 (95% CI: 1.62–1.79; P<0.00001) for OS and 1.75 (95% CI: 1.31–2.33; P<0.0001) PFS of patients with solid tumors (46). Other investigators have studied the prognostic value of serum LDH in thymic carcinoma. Wu and colleagues (22) reported that serum LDH level was an independent prognostic factor for both OS and PFS. We did not find an independent relationship of LDH with OS in our patients, but that might be explained by differences in the patient series. Wu et al. evaluated 90 patients with advanced thymic carcinoma (Masaoka stages III and IV), but only 62 Masaoka stage III and IV patients were included in our study. Thus, high serum LDH may be an independent predictor of OS in patients with advanced thymic carcinoma. In addition, patients in our series underwent total or subtotal surgical resection, but only 40 of 90 patients (44%) studied by Wu et al. underwent surgical resection and the other patients were treated by chemotherapy and/or radiotherapy only. Therefore, the differences in stage of disease and treatment methods may account for the difference in results.
The rarity of thymic carcinoma limited the size of our patient sample. Nevertheless, it was the largest patient series to demonstrate a correlation between serum LDH levels and prognosis of thymic carcinoma. It was also limited by being a retrospective study that evaluated clinical data from only one institution, which may lead to selection bias. Finally, the clinical effects of different LDH subunits or isoenzymes in predicting survival were not studied due to the lack of laboratory data. Further studies are needed to evaluate the predictive value of various LDH isoforms or isoenzymes in thymic carcinoma.
Conclusions
High serum LDH level (>225 U/L) was an independent marker of poor prognosis in thymic carcinoma patients, indicating decreased PFS. It was also significantly associated with advanced Masaoka stage and decreased OS, but was not an independent predictor of death after surgical resection. Masaoka stage and complete resection were also independent prognostic factors for thymic carcinoma. We recommend use of preoperative serum LDH level in discussing treatment options and possible outcomes with patients. Serum LDH may also supplement the Masaoka staging system.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee board of Cancer Hospital, Chinese Academy of Medical sciences (NO. 16-012/1164).
References
- Carter BW, Marom EM, Detterbeck FC. Approaching the patient with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol 2014;9:S102-9. [Crossref] [PubMed]
- Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-12. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Eng TY, Fuller CD, Jagirdar J, et al. Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys 2004;59:654-64. [Crossref] [PubMed]
- Yano M, Sasaki H, Yokoyama T, et al. Thymic carcinoma: 30 cases at a single institution. J Thorac Oncol 2008;3:265-9. [Crossref] [PubMed]
- Okereke IC, Kesler KA, Freeman RK, et al. Thymic carcinoma: outcomes after surgical resection. Ann Thorac Surg 2012;93:1668-72; discussion 1672-3.
- Rajan A, Giaccone G. Chemotherapy for thymic tumors: induction, consolidation, palliation. Thorac Surg Clin 2011;21:107-14. viii. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029-33. [Crossref] [PubMed]
- Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 2010;10:267-77. [Crossref] [PubMed]
- Thorne JL, Campbell MJ. Nuclear receptors and the Warburg effect in cancer. Int J Cancer 2015;137:1519-27. [Crossref] [PubMed]
- Brown JE, Cook RJ, Lipton A, et al. Serum lactate dehydrogenase is prognostic for survival in patients with bone metastases from breast cancer: a retrospective analysis in bisphosphonate-treated patients. Clin Cancer Res 2012;18:6348-55. [Crossref] [PubMed]
- Bar J, Spencer S, Morgan S, et al. Correlation of lactate dehydrogenase isoenzyme profile with outcome in patients with advanced colorectal cancer treated with chemotherapy and bevacizumab or cediranib: Retrospective analysis of the HORIZON I study. Clin Colorectal Cancer 2014;13:46-53. [Crossref] [PubMed]
- Zhao Z, Han F, Yang S, et al. The clinicopathologic importance of serum lactic dehydrogenase in patients with gastric cancer. Dis Markers 2014;2014:140913.
- Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol 2012;30:3402-7. [Crossref] [PubMed]
- Faloppi L, Scartozzi M, Bianconi M, et al. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer 2014;14:110. [Crossref] [PubMed]
- Jin Y, Ye X, Shao L, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer 2013;49:1619-26. [Crossref] [PubMed]
- Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline--Update 2012. Eur J Cancer 2012;48:2375-90. [Crossref] [PubMed]
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-100, 101.e1-2.
- Weksler B, Dhupar R, Parikh V, et al. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299-303. [Crossref] [PubMed]
- Wu JX, Chen HQ, Shao LD, et al. Long-term follow-up and prognostic factors for advanced thymic carcinoma. Medicine (Baltimore) 2014;93:e324. [Crossref] [PubMed]
- Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer 2015;121:1008-16. [Crossref] [PubMed]
- Racker E, Spector M. Warburg effect revisited: merger of biochemistry and molecular biology. Science 1981;213:303-7. [Crossref] [PubMed]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759-67. [Crossref] [PubMed]
- Ferreira LM, Hebrant A, Dumont JE. Metabolic reprogramming of the tumor. Oncogene 2012;31:3999-4011. [Crossref] [PubMed]
- Peppicelli S, Bianchini F, Calorini L. Metabolic reprogramming as a continuous changing behavior of tumor cells. Tumour Biol 2015;36:5759-62. [Crossref] [PubMed]
- Philip B, Ito K, Moreno-Sánchez R, et al. HIF expression and the role of hypoxic microenvironments within primary tumours as protective sites driving cancer stem cell renewal and metastatic progression. Carcinogenesis 2013;34:1699-707. [Crossref] [PubMed]
- Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006;441:437-43. [Crossref] [PubMed]
- Kato Y, Ozawa S, Miyamoto C, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int 2013;13:89. [Crossref] [PubMed]
- Stern R, Shuster S, Neudecker BA, et al. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res 2002;276:24-31. [Crossref] [PubMed]
- Williams AC, Collard TJ, Paraskeva C. An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: implications for clonal selection during colorectal carcinogenesis. Oncogene 1999;18:3199-204. [Crossref] [PubMed]
- Milovanova TN, Bhopale VM, Sorokina EM, et al. Lactate stimulates vasculogenic stem cells via the thioredoxin system and engages an autocrine activation loop involving hypoxia-inducible factor 1. Mol Cell Biol 2008;28:6248-61. [Crossref] [PubMed]
- Vélez J, Hail N Jr, Konopleva M, et al. Mitochondrial uncoupling and the reprograming of intermediary metabolism in leukemia cells. Front Oncol 2013;3:67. [Crossref] [PubMed]
- Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 2015;17:351-9. [Crossref] [PubMed]
- Peppicelli S, Bianchini F, Calorini L. Extracellular acidity, a "reappreciated" trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev 2014;33:823-32. [Crossref] [PubMed]
- Markert CL. Lactate dehydrogenase isozymes: dissociation and recombination of subunits. Science 1963;140:1329-30. [Crossref] [PubMed]
- Maekawa M. Lactate dehydrogenase isoenzymes. J Chromatogr 1988;429:373-98. [Crossref] [PubMed]
- Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett 2015;358:1-7. [Crossref] [PubMed]
- Jurisic V, Radenkovic S, Konjevic G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv Exp Med Biol 2015;867:115-24. [Crossref] [PubMed]
- Yao Y, Wang H, Li B. LDH5 overexpression is associated with poor survival in patients with solid tumors: a meta-analysis. Tumour Biol 2014;35:6973-81. [Crossref] [PubMed]
- von Eyben FE. A systematic review of lactate dehydrogenase isoenzyme 1 and germ cell tumors. Clin Biochem 2001;34:441-54. [Crossref] [PubMed]
- Arora R, Schmitt D, Karanam B, et al. Inhibition of the Warburg effect with a natural compound reveals a novel measurement for determining the metastatic potential of breast cancers. Oncotarget 2015;6:662-78. [Crossref] [PubMed]
- Nagle SJ, Woo K, Schuster SJ, et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol 2013;88:890-4. [Crossref] [PubMed]
- Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837-42. [Crossref] [PubMed]
- Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol 2015;54:961-70. [Crossref] [PubMed]