Association of CHEK2 polymorphisms with the efficacy of platinum-based chemotherapy for advanced non-small-cell lung cancer in Chinese never-smoking women
Introduction
In China, lung cancer is the most common cause of the deaths by malignancies. Studies show that the incidence of lung cancer is closely related to the ability to repair DNA damage caused by environmental exposure and other factors. Non-small-cell lung cancer (NSCLC) accounts for 80% of all lung cancer cases. As first-line therapy for advanced NSCLC, platinum-based combination chemotherapy has the effect of enhancing cancer patients’ overall survival (OS) and quality of life (1). However, different groups of patients still have different prognoses. It is essential to find precise and effective biomarkers in order to establish personal treatment regimens for each patient. There is arising overall trend toward an increasing incidence and mortality of NSCLC among females, especially in those who never smoked (2). Previous studies have researched the prognosis factors in lung cancer for women, including performance status (PS) and clinical stages of the disease (3).
Single nucleotide polymorphisms (SNPs) are common in the human genome. There is growing evidence that demonstrates that the presence of some SNPs can predict NSCLC patients’ response to chemotherapy (4-7). Evidence for genetic susceptibility to lung cancer will increase the chance to study prognosis factors for never-smoking women with NSCLC receiving chemotherapy.
There are some potential genes to be as the prediction biomarker for cancer till now, such as BRCA1 (8), XRCC1 (9,10), ABCB1 (11), and NDRG4 (12). High-lighting BRCA1 promoter methylation may be a biomarker for effect and better prognosis of DNA damaging agents for breast cancer (8), XRCC1 genetic variants may be the markers for predicting lung cancer susceptibility (9) and are associated to the OS of advanced NSCLC patients treated by gemcitabine/platinum (10). ABCB1 C3435T gene polymorphism may as a potential biomarker of progress free survival in breast cancer patients (11). And as highly expressed methylated NDRG4 gene in colorectal cancer (CRC) patients, the detection of methylated NDRG4 could be used as a novel diagnostic technique for CRC (12).
Since our team has reported some articles about the relationship between treatments efficacy and SNPs in different genes, such as RB1 (13), WEE1 (7), etc. And WEE1 as a G2/M checkpoint kinase can induce G2/M cell cycle arrest in the response to DNA damage. Cell cycle checkpoint kinase 2 (CHEK2) also is a G2/M checkpoint kinase, so we focused on it after WEE1 SNPs research selectively. The checkpoint kinase 2 (CHK2, or CHEK2) gene on chromosome 22 is still playing an important role in tumor suppression (14). DNA damage can cause CHEK2 phosphorylation (15), activated CHEK2 can lead to phosphorylation of the CDC25 family, BRCA1, p53, and other similar functional effectors in order to start the cell cycle checkpoint regulation (15-17). Results from previous studies have shown a relationship between the CHEK2 mutation and an increased risk for lung cancer (18,19), breast cancer (20,21), prostate cancer (22,23), colorectal cancer (24,25) and other cancers (26,27). In addition, there was a relationship between the decreased risk of endometrial cancer and the rs8135424 CHEK2 SNP (28). And there was a significant risk association between CHEK2 SNP rs17507066 and serous epithelial ovarian cancer (29). There are few previous studies which reported about the relationship about CHEK2 SNPs with lung cancer, especially the rs4035540 in CHEK2. We have added a table to summary about the lung cancer related SNPs CHEK2 gene (Table S1).
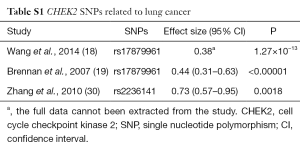
Full table
However, for NSCLC never-smoking female patients receiving platinum-based doublet chemotherapy, there is not enough convincing evidence to prove that a relationship between the CHEK2 genetic polymorphisms and both the prognosis as well as the chemotherapeutic toxicity exists.
In this study, we have analyzed the association between four SNPs in the CHEK2 gene and the efficacy of chemotherapy, as well as the toxicity. We analyzed the data collected from 272 advanced NSCLC never-smoking female patients to try to find the new research direction to predict survival, efficacy, and/-or toxicity for Chinese never-smoking females with NSCLC.
Methods
Patients
In this study, we enrolled 272 female patients who had been diagnosed with clinical stages III–IV NSCLC and were receiving platinum-based (carboplatin or cisplatin) chemotherapy. Enrolled patients were from Shanghai Pulmonary Hospital, Shanghai Zhongshan Hospital, Shanghai Chest Hospital, and Shanghai Changhai Hospital (all China) and would provide their written informed consent before being included in this study. Detailed inclusion criteria included several specific conditions: (I) stages III–IV NSCLC confirmed by at least one diagnostic criteria; (II) inoperable only; (III) platinum-based (cisplatin or carboplatin) chemotherapy as the first-line treatment; (IV) confirmed primary NSCLC by the histological test; (V) the Eastern Cooperative Oncology Group (ECOG) (30) performance status (PS) from 0 to 2; (VI) no history of cancer in other organs; (VII) never received chemotherapy treatment previously; and (VIII) never smoking.
The ethics committee of Shanghai Pulmonary Hospital has approved this study. We have an approval number 2009FK31 from the Institution. Peripheral blood sample collection and the epidemiological information collection all had the informed consent of participants complying with the provisions of the ethics.
Treatment schedules and data collection
All enrolled patients received first-line platinum-based doublet chemotherapy with one of the following double chemotherapy regimens for 2 to 6 cycles: (I) carboplatin or cisplatin plus vinorelbine (NP/NC); (II) carboplatin or cisplatin plus gemcitabine (GP/GC); (III) carboplatin or cisplatin plus paclitaxel (TP/TC); or (IV) carboplatin or cisplatin plus docetaxel (DP/DC).
Following the Response Evaluation Criteria in Solid Tumors (RECIST) criteria 1.1 (31), patients’ responses to platinum-based therapy were assessed after the first two chemotherapy cycles. All responses were re-assessed at least four weeks after initial assessment using the same criteria. For data analyses, subjects achieving a stable disease (SD), partial response (PR) or complete responses (CRs) were grouped as responders; subjects with progressive disease (PD) were considered non-responders. OS was defined as the first day the patients received chemotherapy treatments to the final follow-up or day of death.
Following the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 3.0 (32), chemotherapy toxicity was evaluated twice weekly. A toxicity grade of 3 or 4 recorded during the initial two cycles of therapy was collected for analysis.
Tag SNPs selection and genotyping procedure
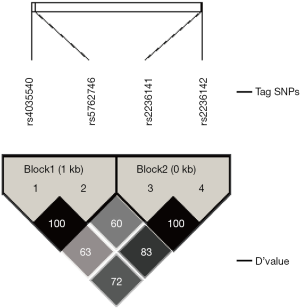
Four tag SNPs (rs4035540, rs5762746, rs2236141, and rs2236142) from the gene region (including 2 kb upstream) of the human CHEK2 (54163bp, 22q12.1) were selected from the Han Chinese population in Beijing (HCB) data downloaded from HapMap SNP databases (http://www.hapmap.org/) (Table 1). The tag SNPs were selected with a cutoff of 0.05 of a minor allele frequency (MAF) by Haploview (33) (http://www.broadinstitute.org/haploview/) or Hardy-Weinberg equilibrium P values <0.05 in the enrolled population. Haplotype blocks were concluded by the methods showed by Gabriel et al. (34).
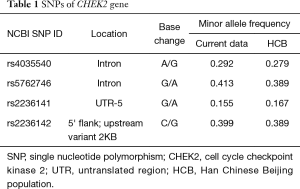
Full table
We have collected the blood 3 to 5 milliliters by anticoagulant blood collection tube from each patient before the chemotherapy. And 400 microliters of blood were used for DNA extraction by the Human QIAamp DNA Blood Maxi Kit (Qiagen GmbH, Hilden, Germany). DNA has been checked by NanoDrop 2000 (Thermo Scientific, USA). When the value of OD 260/280 nm was in the range from 1.8 to 2, we indicated that the DNA was suitable for high-throughput sequencing.
In order not to affect the statistical efficiency, we used MAF >0.05, genotyping call rate >0.95 and the GenCall score >0.2 as the filter criteria for SNP genotyping by using the iSelect HD Bead-Chip (Illumina, CA, USA). The silica beads with 3.1 µm diameter and oligonucleotide probes were made at first. Each bead could be combined with thousands of the same probes at the 5' end of sequences. With the use of microporous etched optical fiber technology on a chip, we could easily handle and add the beads to the chip. Beads were combined with fiber microporous in a disorderly manner. Only one bead was actually placed in each of the microprobe. Results could be read with the Illumina BeadScan machine.
Statistical analysis
SPSS Statistics 20.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. The OS of the enrolled patients was examined by the Kaplan-Meier and log-rank tests. Multivariate analyses were carried out using the Cox proportional hazard model by adjusting for clinical characteristics, such as age, TNM stage, pathologic types, adjuvant therapy and PS. Differences with P<0.05 were considered statistically significant. Multivariate logistic regression analysis was used to estimate the response to therapy and the toxicity risk for each SNP with adjustment for clinical characteristics.
Results
Association between clinical characteristics and survival in enrolled patients treated with platinum-based chemotherapy
The cohort consisted of 272 NSCLC non-smoking female patients with a median age of 57 years, ranging from 26 to 80 years old. All patients had been diagnosed as IIIa/IIIb or IV clinical stage according to the ECOG PS 1 or 2.
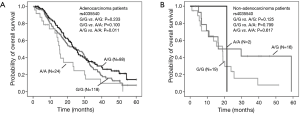
The relationship of clinical characteristics with OS was analyzed using Kaplan-Meier curve test. No significant correlation was observed between the OS and pathologic types, adjuvant therapy, or ECOG PS (score 1 or 2) (Table 2). Since we focus on never-smoking female NSCLC patients, the clinical variable which may have effects on OS is the factor analyzed. The log-rank test suggested that younger patients (≤57 years old) had a significant better OS than elderly ones (Table 2, Log-Rank P=0.012; Figure 1A) and patients with TNM stage III had a significantly better OS than those with stage IV (Table 2, Log-Rank P=0.021; Figure 1B).
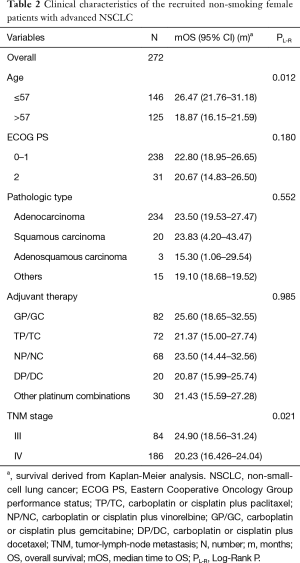
Full table
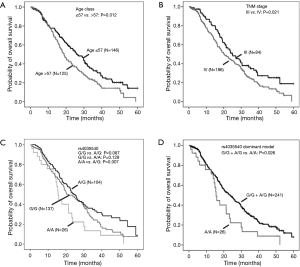
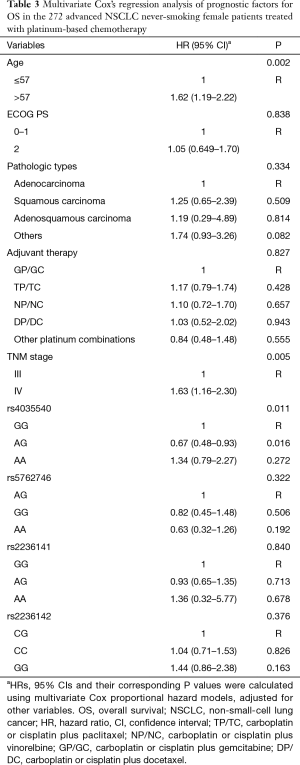
Furthermore, the multivariate Cox’s proportional hazards regression analysis suggested that age classified according to a cutoff of 57 years old (Table 3, HR =1.62, 95% CI: 1.19–2.22; P=0.002) and TNM stage (Table 3, HR =1.63, 95% CI: 1.16–2.30; P=0.005) were independent predictive factors.
Full table
Association between CHEK2 tag SNPs and survival in enrolled patients treated with platinum-based chemotherapy
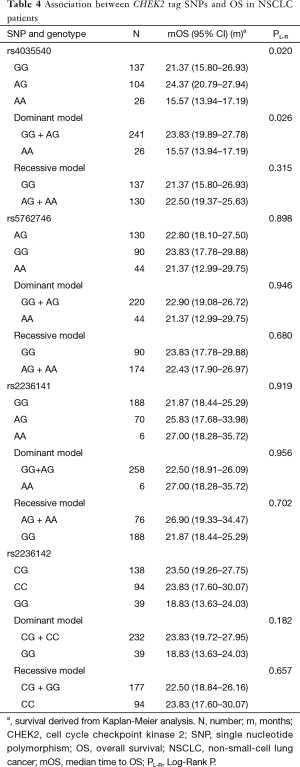
Analysis of the relationship by the log rank test between SNPs and OS showed that CHEK2 rs4035540 (Table 4, Log-Rank P=0.020; Figure 1C) was significantly related NSCLC patients’ OS. Furthermore, we also investigated the association between OS and both the CHEK2 rs4035540 dominant model, as well as recessive model. As a result, the dominant model was also found to be a significant factor contributing to the OS of the enrolled patients treated with platinum-based chemotherapy (Table 4, Log-Rank P=0.026; Figure 1D).
Full table
Using multivariate Cox proportional hazard models, patients with rs4035540 A/G genotype had a significantly better OS than those with the G/G genotype (Table 3, HR =0.67, 95% CI, 0.48–0.93; P=0.016).
Association between CHEK2 tag SNPs and survival in NSCLC female patients classified by pathologic types
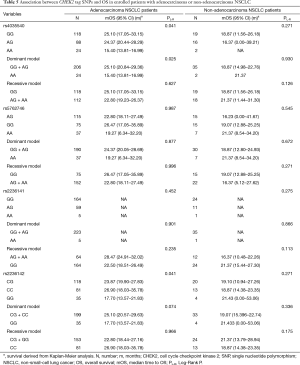
We further investigated the influence of SNPs on OS stratified by pathologic types (Table 5). The results suggested that the above rs4035540 (Log-Rank P=0.041) and rs2236142 (Log-Rank P=0.041), as well as the rs4035540 dominant model (Log-Rank P=0.025) were significantly associated with OS in non-smoking female patients with lung adenocarcinoma (Table 5, Figure 2).
Full table
Toxicity
After analyzing the possible association of chemotherapy toxicity with different treatment regimens, it was shown that CHEK2 rs4035540 was significantly associated with gastrointestinal toxicity. There was a significantly higher toxicity in the A/A genotype group than the G/G genotype group (adjusted OR =3.83, 95% CI, 1.36–10.56; P=0.009) (Table 6).
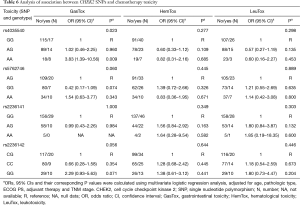
Full table
Chemotherapy response
There were no significant associations between chemotherapy treatments and genetic variations (Table S2). At the same time, we also found there was not any significant relationship between chemotherapy response and genetic variations grouped by the different regimens (Table S3).
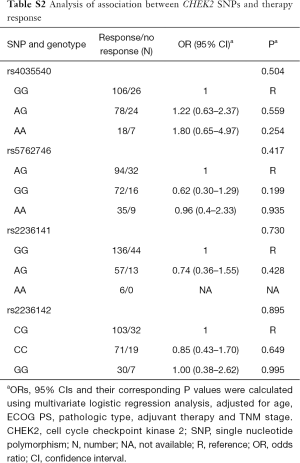
Full table

Full table
Haploview analysis of CHEK2 tag SNPs
Among the four SNPs, the rs4035540 and rs5762746 were in strong LD (Linkage Disequilibrium) (│D’│=100%, r2=0.563). And rs2236141 and rs2236142 were in LD (│D’│=100%, r2=0.121). The results could be showed from the Haploview Software directly (Figure S1). Since only one tag SNP had the significance with the OS, there was no real meaning for analyzing the linkage disequilibrium between different SNPs.
Discussion
CHEK2 plays an essential role in the signaling pathway for DNA damage and cell checkpoint regulation. In order to maintain genomic stability when DNA damage occurs, CHEK2 is activated via phosphorylation of downstream genes (14). The cell cycle checkpoint is an important mechanism in maintaining genomic stability. A DNA damage checkpoint can detect the DNA damage in the cell cycle and lead to cell cycle arrest to provide enough time to repair the damage (35). Many studies have reported that CHEK2 gene mutations could increase the risk of some cancers (20,22,27,36-38). Many investigators have researched this area with special emphasis on CHEK2 1100delC.
There is a growing realization that genetic polymorphisms not only have effects on cancer development, but also on its prognosis. Numerous SNPs in CHEK2 have been screened to reveal possible relationships with various cancers. In a study by Lawrenson et al. (29), the results indicated a significant risk association between CHEK2 SNP rs17507066 and serous epithelial ovarian cancer (P=4.74E-7). Meanwhile, Gu et al. have reported that (39) about CHEK2 gene with the results showing that the rs738722 C/T polymorphism and the rs2236142 G/C polymorphism might be protective factors against the risk of lymph node metastasis of esophageal cancer in the Chinese population. However, whether the polymorphism of the CHEK2 gene is associated with the efficacy of platinum-based doublet chemotherapy in never-smoking NSCLC female patients has not yet been investigated.
Our study has provided evidence for a relationship between the CHEK2 genetic polymorphisms and efficacy of platinum-based doublet chemotherapy in never-smoking Chinese NSCLC female patients. The A/G allele in rs4035540 in CHEK2 has been verified to be an independent protective factor for the prognosis for never-smoking female NSCLC patients. At the same time, TNM stages and age classes could also be the independent factors for predicting prognosis for these Chinese patients. Different histological types of NSCLC may have different biological behavior. After the subgroup analysis classified by pathologic types, we found that CHEK2 rs4035540 and rs2236142 may be significant in predicting OS in the never-smoking female patients lung adenocarcinoma group. Therefore, based on the findings of this study, we consider that CHEK2 polymorphisms may affect the efficacy of platinum-based chemotherapy, and may play valuable roles in predicting the prognosis for NSCLC with never-smoking female patients.
In addition, although platinum-based doublet chemotherapy has been proved to be effective for NSCLC patients, adverse side effects still exist because of the platinum-related DNA damage (40). We analyzed the incidence of drug toxicities, including gastrointestinal, hematological and leukotoxicities. As a result, in our patients, we found that CHEK2 rs4035540 A/A genotype group demonstrated higher gastrointestinal toxicity after treatment than the G/G genotype group. This finding suggests that it may help to identify patient subgroups with a strong risk for drug toxicity by testing for the presence of these polymorphisms for the selection of appropriate chemotherapy for the treatment of female NSCLC.
Our results suggest that genetic variations in the CHEK2 gene may play a role in predicting the toxicity and prognosis of NSCLC. The clinical significance of CHEK2 rs4035540 polymorphism in platinum-based chemotherapy of NSCLC has not been reported before. The screening of tagged SNPs of CHEK2 in this study may bring a new evidence of the importance of CHEK2 in NSCLC and the efficacy of treatments to clinical.
However, CHEK2 rs4035540 is located in the intron area of the CHEK2 gene. After the Haploview analysis, we made a very bold speculation that it may modify the expression levels of CHEK2 gene based on linkage disequilibrium with multiple SNPs. And this speculation about the complex signaling pathway of which CHEK2 is involved is our following plan to research. Determining whether the CHEK2 rs4035540 A/G genotype can be used a biomarker for the option of platinum-based regimen in NSCLC patients and the detailed mechanism are worth further studying in the future. The additional follow-up studies with a larger patient sample that includes different ethnic populations are warranted.
Conclusions
We demonstrated for the first time that polymorphisms in the CHEK2 gene may predict the clinical outcomes for advanced Chinese never-smoked NSCLC female patients following platinum-based doublet chemotherapy. Our study provides evidence of a useful molecular research direction which may be easily applied in clinical situation. Consequently, in addition to the clinical stages and the pathologic types classified by lung adenocarcinoma, it may help identify patient subgroups with the risk of poor diseases outcomes by testing for the presence of these polymorphisms; the information can be used to tailor personal therapies in certain populations. The mechanisms for the effects of these SNPs on CHEK2 biologic functions remain to be clarified in further studies to understand the role of the CHEK2 gene in determining NSCLC outcomes after platinum-based doublet chemotherapy.
Acknowledgements
Funding: This work was funded by National Natural Science Foundation of China (No. 81572269), Science and Technology Commission of Shanghai Municipality (No. 14411966400, No. 134119a3400), Shanghai Health Bureau Foundation (No. 201440397, XYQ2013115) and Med-Engineering Interdisciplinary Research Foundation (No. YG2015MS71), Shanghai Jiaotong University (No. 201440397).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of Shanghai Pulmonary Hospital (No. 2009FK31) and written informed consent was obtained from all patients.
References
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol 2008;26:4617-25. [Crossref] [PubMed]
- Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 2008;5:e185. [Crossref] [PubMed]
- Radzikowska E, Głaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol 2002;13:1087-93. [Crossref] [PubMed]
- Grimminger PP, Stöhlmacher J, Vallböhmer D, et al. Prognostic Significance and Clinicopathological Associations of COX-2 SNP in Patients with Nonsmall Cell Lung Cancer. J Oncol 2009;2009:139590.
- Yin Z, Zhou B, He Q, et al. Association between polymorphisms in DNA repair genes and survival of non-smoking female patients with lung adenocarcinoma. BMC Cancer 2009;9:439. [Crossref] [PubMed]
- Lai LC, Tsai MH, Chen PC, et al. SNP rs10248565 in HDAC9 as a novel genomic aberration biomarker of lung adenocarcinoma in non-smoking women. J Biomed Sci 2014;21:24. [Crossref] [PubMed]
- Liu D, Wu C, Jiao Y, et al. WEE1 kinase polymorphism as a predictive biomarker for efficacy of platinum-gemcitabine doublet chemotherapy in advanced non-small cell lung cancer patients. Sci Rep 2015;5:11114. [Crossref] [PubMed]
- Zhu X, Shan L, Wang F, et al. Hypermethylation of BRCA1 gene: implication for prognostic biomarker and therapeutic target in sporadic primary triple-negative breast cancer. Hypermethylation of BRCA1 gene: implication for prognostic biomarker and therapeutic target in sporadic primary triple-negative breast cancer. Breast Cancer Res Treat 2015;150:479-86. [Crossref] [PubMed]
- Zhu DQ, Zou Q, Hu CH, et al. XRCC1 genetic polymorphism acts a potential biomarker for lung cancer. Tumour Biol 2015;36:3745-50. [Crossref] [PubMed]
- Liao WY, Shih JY, Chang GC, et al. Genetic polymorphism of XRCC1 Arg399Gln is associated with survival in non-small-cell lung cancer patients treated with gemcitabine/platinum. J Thorac Oncol 2012;7:973-81. [Crossref] [PubMed]
- Madrid-Paredes A, Cañadas-Garre M, Sánchez-Pozo A, et al. ABCB1 C3435T gene polymorphism as a potential biomarker of clinical outcomes in HER2-positive breast cancer patients. Pharmacol Res 2016;108:111-118. [Crossref] [PubMed]
- Xiao W, Zhao H, Dong W, et al. Quantitative detection of methylated NDRG4 gene as a candidate biomarker for diagnosis of colorectal cancer. Oncol Lett 2015;9:1383-7. [PubMed]
- Liu D, Xu W, Zhang ZW, et al. RB1 polymorphism contributes to the efficacy of platinum-taxanes in advanced squamous cell lung cancer. Asian Pac J Cancer Prev 2015;16:775-81. [Crossref] [PubMed]
- Ahn J, Urist M, Prives C., et al. The Chk2 protein kinase. DNA Repair (Amst) 2004;3:1039-47. [Crossref] [PubMed]
- Falck J, Mailand N, Syljuåsen RG, et al. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 2001;410:842-7. [Crossref] [PubMed]
- Stevens C, Smith L, La Thangue NB. Chk2 activates E2F-1 in response to DNA damage. Nat Cell Biol 2003;5:401-9. [Crossref] [PubMed]
- Vaziri C, Saxena S, Jeon Y, et al. A p53-dependent checkpoint pathway prevents rereplication. Mol Cell 2003;11:997-1008. [Crossref] [PubMed]
- Wang Y, McKay JD, Rafnar T, et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat Genet 2014;46:736-41. [Crossref] [PubMed]
- Brennan P, McKay J, Moore L, et al. Uncommon CHEK2 mis-sense variant and reduced risk of tobacco-related cancers: case control study. Hum Mol Genet 2007;16:1794-801. [Crossref] [PubMed]
- Weischer M, Nordestgaard BG, Pharoah P, et al. CHEK2*1100delC heterozygosity in women with breast cancer associated with early death, breast cancer-specific death, and increased risk of a second breast cancer. J Clin Oncol 2012;30:4308-16. [Crossref] [PubMed]
- Weischer M, Bojesen SE, Tybjaerg-Hansen A, et al. Increased risk of breast cancer associated with CHEK2*1100delC. J Clin Oncol 2007;25:57-63. [Crossref] [PubMed]
- Hale V, Weischer M, Park JY. CHEK2 (*) 1100delC Mutation and Risk of Prostate Cancer. Prostate Cancer 2014;2014:294575.
- Zheng L, Wang F, Qian C, et al. Unique substitution of CHEK2 and TP53 mutations implicated in primary prostate tumors and cancer cell lines. Hum Mutat 2006;27:1062-3. [Crossref] [PubMed]
- Wasielewski M, Vasen H, Wijnen J, et al. CHEK2 1100delC is a susceptibility allele for HNPCC-related colorectal cancer. Clin Cancer Res 2008;14:4989-94. [Crossref] [PubMed]
- Xiang HP, Geng XP, Ge WW, et al. Meta-analysis of CHEK2 1100delC variant and colorectal cancer susceptibility. Eur J Cancer 2011;47:2546-51. [Crossref] [PubMed]
- Złowocka E, Cybulski C, Górski B, et al. Germline mutations in the CHEK2 kinase gene are associated with an increased risk of bladder cancer. Int J Cancer 2008;122:583-6. [Crossref] [PubMed]
- Teodorczyk U, Cybulski C, Wokołorczyk D, et al. The risk of gastric cancer in carriers of CHEK2 mutations. Fam Cancer 2013;12:473-8. [Crossref] [PubMed]
- O'Mara TA, Ferguson K, Fahey P, et al. CHEK2, MGMT, SULT1E1 and SULT1A1 polymorphisms and endometrial cancer risk. Twin Res Hum Genet 2011;14:328-32. [Crossref] [PubMed]
- Lawrenson K, Iversen ES, Tyrer J, et al. Common variants at the CHEK2 gene locus and risk of epithelial ovarian cancer. Carcinogenesis 2015;36:1341-53. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. [Crossref] [PubMed]
- Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005;21:263-5. [Crossref] [PubMed]
- Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science 2002;296:2225-9. [Crossref] [PubMed]
- Casimiro MC, Crosariol M, Loro E, et al. Cyclins and cell cycle control in cancer and disease. Genes Cancer 2012;3:649-57. [Crossref] [PubMed]
- Wasielewski M, den Bakker MA, van den Ouweland A, et al. CHEK2 1100delC and male breast cancer in the Netherlands. Breast Cancer Res Treat 2009;116:397-400. [Crossref] [PubMed]
- Tung N, Silver DP. Chek2 DNA damage response pathway and inherited breast cancer risk. J Clin Oncol 2011;29:3813-5. [Crossref] [PubMed]
- Suchy J, Cybulski C, Wokołorczyk D, et al. CHEK2 mutations and HNPCC-related colorectal cancer. Int J Cancer 2010;126:3005-9. [PubMed]
- Gu H, Qiu W, Wan Y, et al. Variant allele of CHEK2 is associated with a decreased risk of esophageal cancer lymph node metastasis in a Chinese population. Mol Biol Rep 2012;39:5977-84. [Crossref] [PubMed]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 2007;33:9-23. [Crossref] [PubMed]
- Zhang S, Lu J, Zhao X, et al. A variant in the CHEK2 promoter at a methylation site relieves transcriptional repression and confers reduced risk of lung cancer. Carcinogenesis 2010;31:1251-8. [Crossref] [PubMed]


