Systemic therapy for echinoderm microtubule-associated protein-like 4 anaplastic lymphoma kinase non-small cell lung cancer brain metastases
The fusion between echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) occurs in 4−5% of non-small cell lung cancer (NSCLC) patients (1). Crizotinib (Xalkori®), the first approved small-molecule kinase inhibitor, has been proven to be effective in treatment of advanced ALK-positive NSCLC, increasing progression-free survival (PFS), response rates, and overall quality of life (2). Approximately 15% to 35% of ALK-positive NSCLC patients develop brain metastases, ultimately resulting in high mortality rates (3). Although crizotinib has been proven to show successful treatment of extracranial tumor sites, its intracranial effectiveness has yet to be studied extensively (4).
Previously, a clinical study conducted by Costa et al. retrospectively analyzed crizotinib and intracranial disease control rates (IC-DCR) based on the PROFILE 1005 and 1007 (4). PROFILE 1005 was a phase II trial that evaluated the efficacy and safety of crizotinib in ALK-positive NSCLC patients, and PROFILE 1007 was a phase III trial investigating crizotinib as a second-line treatment method in similar patients. The results of the studies revealed that crizotinib use was associated with aim improved IC-DCR of 62% (95% confidence interval, 54% to 70%) at 12 weeks in patients with previously-treated brain metastases.
A similar study, PROFILE 1014, is an ongoing open-label, random, phase III trial conducted by Solomon et al. comparing crizotinib with standard chemotherapy in 343 patients with advanced ALK-positive NSCLC who have received no previous systemic treatment, studying the intracranial efficacy of this therapeutic protocol (5). Patients in the crizotinib arm received oral doses twice daily (250 mg), while those allocated to the chemotherapy arm received a maximum of six cycles of pemetrexed (500 mg/m2) and cisplatin (75 mg/m2)/carboplatin (area under the curve of 5 to 6 mg·min/mL), delivered intravenously every three weeks. Intracranial efficacy was determined by comparing CT and MRI scans every 6 weeks for BM patients and every 12 weeks for patients without brain metastases and was evaluated based on PFS, overall response rate (ORR), overall survival (OS), intracranial time-to-tumor progression (IC-TTP), and intracranial progressive disease (IC-PD).
In a recent update on PROFILE 1014, Solomon and colleagues reported that crizotinib led to a significantly higher PFS in both subgroups of patients with tumor brain metastases (tBM) and without tBM [tBM present: hazard ratio (HR), 0.40; P<0.001; median, 9.0 months with crizotinib vs. 4.0 months with chemotherapy; BM absent: HR, 0.51; P<0.001; median, 11.1 vs. 7.2 months, respectively] and a significant increase in IC-DCR in patients with tBM at 12 weeks (85% with crizotinib vs. 45% with chemotherapy, respectively; P<0.001) and 24 weeks (56% vs. 25%, respectively; P=0.006) (6).
The authors observation that crizotinib showed efficacy in the brain is an important finding and contributes to a growing literature supporting the notion that systemic agents, including epidermal growth factor receptor (EGFR) inhibitors, other ALK inhibitors and standard chemotherapy, can have efficacy in the treatment of brain metastases including those from NSCLC despite the blood brain barrier (7-9). Brain metastases have been shown to increase the leakiness of the neurovascular basement membrane, and BM as small as 0.5 mm can alter the host neovasculature, potentially increasing the permeability of the blood brain barrier to therapeutic drug penetration (10). Previous clinical studies in chemonaive NSCLC patients with BM undergoing pemetrexed and cisplatin had cerebral response rate of 41.9% and ORR of 34.9% (9). In the case of crizotinib, the evidence is both promising but not fully straightforward. Thus, the brain is disproportionately a site of recurrence.
Likewise, Solomon et al. note that in PROFILE 1014, percentages of patients with the brain as the sole site of PD were higher with crizotinib than with chemotherapy in all groups [intent to treat (ITT) population: 24% vs. 10%, respectively; tBM present: 38% vs. 23%, respectively; BM absent: 19% vs. 6%, respectively] (6). Crizotinib has been noted to have a low CSF-to-plasma ratio, and although the main mechanisms of intracranial limitation of crizotinib are still not fully understood yet, it is believed to be due poor blood–brain barrier penetration and acquired drug resistance due to new mutations, such as in IGF-1R, KRAS, and EGFR (4,8). In the case of ALK altered tumors, there now exist agents that target these mutations, including ceritinib and especially alectinib, which show higher efficacy intra and extra cranially, both as initial and as salvage therapy. In a phase I study, ALK-positive NSCLC patients with crizotinib resistance who were treated with ceritinib showed an ORR of 56% (95% CI, 45–67%) (11). Of the 64 ceritinib-treated patients with brain metastases, median PFS was comparable to that of patients without BM (6.9 vs. 7.0 months, respectively). Another relevant phase I trial involving ceritinib in ALK-positive NSCLC patients, ASCEND I, analyzed 94 patients with BM and revealed that 15 of 19 treatment naive patients and 49 of 75 treatment-experienced patients achieved IC-DCR (12).
Alectinib is another powerful ALK inhibitor that has been shown to increase central nervous system (CNS) activity in crizotinib-resistant patients. A phase II study in ALK-positive NSCLC patients observed an objective response of 48% (13). More recently, J-ALEX, a phase III study comparing alectinib and crizotinib in treatment naive patients, showed an ORR of 85.4% in the alectinib group versus 70.2% in the crizotinib group (14). In patients with brain metastases, the hazard ratio for alectinib versus crizotinib was 0.08 (95% CI, 0.01–0.61). The results of these studies support the possibility that alectinib could likely replace crizotinib as the standard first-line therapy for ALK-positive NSCLC in the future. Additional second generation ALK-inhibitors shown to have efficacy in the brain include brigatinib, PF-06463922, ASP3026, X396, and entrectinib (8). More details on their mechanisms of action as well as current clinical trials and results are listed in Table 1.
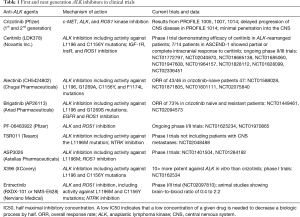
Full table
Other important mechanisms to control CNS disease include targeting pathways downstream of ALK phosphorylation, increasing the permeability of the blood-brain barrier, modifying the tumor microenvironment, and adding concurrent radiotherapy (8). In preclinical NSCLC models, the combination of radiation therapy and crizotinib has been shown to stall tumor growth and increase apoptosis (15). Drugs targeting PI3K, mTOR, IGF-1R, and other pathways in combination with ALK inhibitors have also been shown to have the potential to improve overall outcomes of BM (8).
It must be remembered that the high probability of benefit in the treatment of brain metastases may represent not so much the high probability of efficacy in the brain but the high probability of response of ALK targeting agents in ALK activated NSCLC, whether in the brain or extracranial sites. As drugs become more effective extracranially, they are also more likely to be increasingly effective intracranially. Indeed, these insights are increasingly being integrated into trials in which patients with asymptomatic brain metastases not yet treated with external-beam radiation therapy (XRT) are eligible for study (16). Although usages of stereotactic radiosurgery (SRS) and whole-brain radiation therapy (WBRT) are widely used treatment options for brain metastases that have been shown to be effective in maintaining local CNS control, there is concern over neurotoxicity of treatment, especially in combination with ALK inhibitors (17). Whole brain and SRS offer a high but imperfect probability of benefit at the cost of acute and sustained toxicities. Such interventions are warranted when the cost of failure of efficacy is high and the probability of the alternatives is less. However, with increased probability of efficacy, with some drugs approaching or even surpassing the probability of benefit from whole brain radiation, we are positioned to re-evaluate when to treat with radiation versus the increasingly tenable option of systemic therapy. Since the data from PROFILE 1014 and other prospective studies prove that crizotinib, next-generation ALK inhibitors, and even standard chemotherapy are effective intracranially, it is plausible that in the future, first-line therapy will consist of these options allowing for the postponement of radiation therapy as a last-line therapy.
In conclusion, the latest update on PROFILE 1014 is an important yet not a novel contribution to the ever-growing idea that drug therapy works in the brain. Usage of these drugs, whether it be ALK inhibitors, EGFR TKIs, or chemotherapy, as first-line agents could very well be just as effective as WBRT, systemically and intracranially, with lesser toxicity. The study is not sufficient on its own to support this point; however, it adds great weight in opening up a much-needed conversation on the topic.
Acknowledgements
None.
Footnote
Provenance: This is an invited Commentary commissioned by the Section Editor Long Jiang (Second Affiliated Hospital, Institute of Respiratory Diseases, Zhejiang University School of Medicine, Hangzhou, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Guérin A, Sasane M, Zhang J, et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ 2015;18:312-22. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol 2016;34:2858-65. [Crossref] [PubMed]
- Lee DH, Han JY, Lee HG, et al. Gefitinib as a first-line therapy of advanced or metastatic adenocarcinoma of the lung in never-smokers. Clin Cancer Res 2005;11:3032-7. [Crossref] [PubMed]
- Zhang I, Zaorsky NG, Palmer JD, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol 2015;16:e510-21. [Crossref] [PubMed]
- Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol 2011;22:2466-70. [Crossref] [PubMed]
- Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol 2007;25:2306-12. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-Rearranged Non–Small-Cell Lung Cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Nokihara H, Hida T, Kondo M, et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): Primary results from the J-ALEX study. J Clin Oncol 2016;34:abstr 9008.
- Sun Y, Nowak KA, Zaorsky NG, et al. ALK inhibitor PF02341066 (crizotinib) increases sensitivity to radiation in non-small cell lung cancer expressing EML4-ALK. Mol Cancer Ther 2013;12:696-704. [Crossref] [PubMed]
- Lee DH, Han JY, Kim HT, et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first: result of a randomized pilot study. Cancer 2008;113:143-9. [Crossref] [PubMed]
- Shi W, Dicker AP. CNS Metastases in Patients With Non-Small-Cell Lung Cancer and ALK Gene Rearrangement. J Clin Oncol 2016;34:107-9. [Crossref] [PubMed]


