Prognostic effects of pulmonary hypertension in patients undergoing cardiac resynchronization therapy
Department of Cardiology, Shenyang Northern Hospital, Shenyang 110016, Liaoning, China
|
Original Article
Prognostic effects of pulmonary hypertension in patients undergoing cardiac resynchronization therapy
Department of Cardiology, Shenyang Northern Hospital, Shenyang 110016, Liaoning, China
|
|
Abstract
Background: Aim of this study is to investigate the impact of elevated pulmonary artery systolic pressure (PASP) on
mortality and the clinical outcome after cardiac resynchronization therapy (CRT).
Methods: Ninety-three patients with heart failure were enrolled into this study, and all of them have been treated by
CRT for more than 6 months. Based on the level of preoperative PASP, they were divided into three groups (Group I:
PASP>50mmHg, n=29; Group II: 30mmHg<PASP≤50mmHg, n=17; Group III: PASP≤30mmHg, n=47). Mortality
and the clinical outcome were compared among three groups in a mean follow-up period of 32.01±20.05 months.
Results: ①Eight (28%), one (6%) and eight (17%) patients died in-group I, II and III respectively. Among those
patients, 5 in group I and 1 in group III died of heart failure, while the patient in group II died of sudden death. ②In
all three groups, CRT significantly improved heart function evaluated by NYHA heart function class and 6 minutes
walking distance (6-MWT) (P<0.01). The improvement was more significant in group III than group I (P<0.01). ③
At 3 months after CRT, Left ventricular ejection fraction (LVEF) increased significantly in Group III (P<0.01), but
not in Group I or II (all P>0.05. At 6 months after CRT, LVEF increased significantly in all three groups (all P<0.05).
Conclusion: Elevated PASP has no prognostic effects on heart function improvement in patients undergone CRT.
However, it was associated with worse LV remodeling and increased death due to aggravation of heart failure.
Key words
heart failure; cardiac resynchronization therapy; pulmonary artery systolic pressure; prognosis
J Thorac Dis 2010;2:71-75. DOI: 10.3978/j.issn.2072-1439.2010.02.02.004
|
|
Introduction
The cardiac resynchronization therapy (CRT) has been
used in treating congestive heart failure for 15 years.
Reported clinical trials have shown that CRT is beneficial
for the sever heart failure. The CRT alone or combined
with the medical management has shown evidence in
improving the heart failure symptoms, quality of life,
exercise capacity, and left ventricular (LV) systolic
performance and overall survival time (1-5). CRT has
become a standard therapy in cases of heart failure and
inter-and intra-ventricular conduction disturbances.
Unfortunately, 20~30% of patients do not respond to
CRT (6). The reason for this failure of CRT may be the
poor positioning of LV leads, poor resynchronization of LV and scar, ischemia/hibernation of myocardium.
(7,8,9). Pulmonary hypertension is a frequently found in
patients with congestive heart failure, which is associated
with a worse prognosis in these patients. Adjunctive
measurements, for this group of patients with refractory
symptomatic pulmonary hypertension are needed. This
study aims on efficacy of CRT in patients with cardiac
failure along with pulmonary hypertension.
|
|
Methods
Patient characteristics
Between March 2003 and June 2008, a total 93
consecutive patients with cardiac failure underwent CRT
after failed conventional medical management. There were
76 men and 17 women; with mean age of cohort were 59.4
(range, xx-xx). Twenty-five of these patients had ischemic
heart disease (CAD) and 68 patients presented with
idiopathic dilated cardiomyopathy (DCM). All patients met
the criteria of Ⅰor Ⅱa indication for CRT (10), including
New York Heart Association (NYHA) Class Ⅲ to Ⅳ,
left ventricular end-diastole diameter (LVEDD) >55mm,
left ventricular ejection fraction (LVEF) <35%, mitral
regurgitation and underwent CRT-P/CRT-D implantation.
Based on echocardiographic estimation of pulmonary
artery pressure (PASP), patients were retrospectively
divided into 3 groups. There were 29 patients in group I
with PASP greater than 50mmHg, 17 patients in group
II with PASP greater than 30mmHg but equal or less
50mmHg, and 47 patients in group III with PASP less than
30mmHg (table 1). After the CRT, patients continued their
conventional therapies, including diuretics, angiotensinconverting
enzyme inhibitors, digitalis and b-blockers.
CRT device implantation
A permanent biventricular intravenous pacing systems
were implanted, consistent of 17 patients model 8040,
38 patients with model 8042, 2 patients with model
7272, 4 patients with model 7279, 3 patients with model
7285, 4 patients with Sentry; 2 patients with Medtronic
Inc. model 5510, 21 patients with model V350, ST. Jude
Medical). All implant devices were programmed to
maximize biventricular pacing throughout the ranges of
expected patient’s activity, and to minimize the power
output to prolong the battery life. Further optimization
of atrio-ventricular (AV) delay was adjusted by using
Doppler trans-mitral flow to provide the maximum left
ventricular filling time without compromising cardiac
resynchronization. The AV delay was set at a value that
provided maximum separation of the E and A waves to select the shortest AV delay without compromising the left
atrial contribution to the left ventricular filling. The VV
delay was set at the maximal value of velocity time integral
(VTI).
Echocardiography
Transthoracic 2-dimensional (2D) echocardiography was
performed the day before CRT implantation, 3 months and
6 months after CRT. Patients were imaged in the left lateral
decubitus position. Images were obtained using a 3.5-MHz
transducer, at a depth of 16 cm in the parasternal and apical
views (standard long-axis, 2- and 4-chamber images).
Standard 2D and color Doppler data, triggered to the
QRS complex, were saved in dincine loop format. The LV
volume (from the end-diastolic to the end-systolic volume)
and LVEF were calculated from the conventional apical
2- and 4-chamber images, using the biplane Simpson’s
technique (11).
Follow up
Patients were followed 1, 3 and 6 months after the
procedure and every 6 months thereafter at our outpatient
heart failure clinic. All patients were re-evaluated at 3
months and 6 months after a CRT implantation, which
included the NYHA heart function class, 6-MWT and
echocardiographic parameters (LVEDD, LVEF). A median follow-up in this study was 32.0 months (range, 6-60
months). The mortalities were assessed up to 5 years.
Statistical analysis
Data are expressed as mean SD. Comparisons between
mean values of continuous variables were performed
by a two-sided paired t-test, or an unpaired t-test when
necessary; chi-square test with continuity correction was
used for dichotomous variables. Kaplan-Meier curves for
evaluation of survival rate were established using the logrank
test. For all analyses, p<0.05 was considered to be
statistical significant.
|
|
Results
Mortality
Eight patients (28%) in group I have died. Five of these
patients died of aggressive heart failure, 1 patient died of
AMI, and 2 patients died of sudden death unknown reason.
One patient (6%) died in Group II, and cause of death was
a sudden death. Eight patients (17%) have died group III,
including that 1 died of advanced heart failure, 4 died of
sudden death, and 3 others died of non-cardiac diseases.
There was no statistical difference among group I, II, and
III in terms of total mortality (P>0.05), while mortality
from decomposition of heart failure was significantly
higher in Group I (p<0.01). Figure 1 shows the mortalities
in all three groups.
NYHA heart function
After CRT implantation, all group patients’ NYHA heart
function grades showed significant improvements (P<0.01),
among which patients in Group III demonstrated more significant improvement than those in Group I (Figure 2. A,
P<0.01).
6-MWT
6-MWT increased significantly at postoperative 3 to
6 months in all groups (P<0.05 in Group I, P<0.05~0.01
in Group II, and P<0.01 in Group III), particularly, which
in Group III increased by 130m compared to ones in preoperative
evaluation (P<0.01). 6-MWT measurements
showed a significant improvement in Group III than in
Group I at 3 and 6 months post-implantation (Figure 2. B).
LVEDD
The LVEDD diminished significantly from 71mm
to 66mm at 3-6 months in Group III (P<0.05), but not
significantly different in Group I (from 71mm to 68mm,
P>0.05) and Group II (from 69mm to 66mm, P>0.05).
Figure 2 (C) shows LVEDD changes in all groups.
LVEF
The LVEF improved at 3-6 months in all groups. LVEF
increased from 31 to 39% at 6 months in Group II (P<0.01),
and group III showed a significant increase at 3-6 months,
consisting of from 31 to 39% at 3 months, and from 31
to 44% at 6 months (all P<0.01). The LVEF in Group III
increased more significant than ones in Group I and Group
II at postoperative 6 months. Figure 2 (D) shows LVEF
changes in all groups.
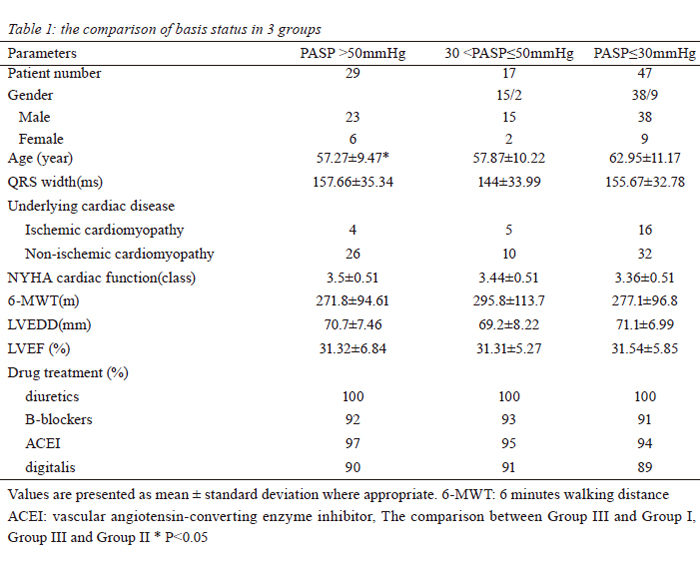 Figure 1 Kaplan-Meier cumulative survival curve among three groups according to PASP. There was no statistical significance among curves (p=0.33).
Figure 2 Panel A: The improvement of NYHA class among groups. Panel B: The difference of 6-MWT among groups. Panel C: The diminution of LVEDD among groups. Panel D: The increase of LVEF among groups. *P<0.05, ** P<0.01 the postoperative compared with the preoperative in the group. # P<0.05, ## P<0.01 vs. same time among groups.
|
|
Discussion
The systolic function of the heart depends on the
concordant contraction of each compartment of the heart.
The poor concordant contraction can reflect the disorder
of myocardial movement and function from the change
of myocardial construction (12). Pulmonary hypertension
is the pathologic status of pulmonary artery pressure
over normal from all reasons. Continuation of elevating
pulmonary artery pressure can lead to increase of right
ventricular filling pressure resulting in the thickening,
degenerating, and fibrosis of the myocardium, further
causing the right ventricular (RV) dysfunction and right
heart failure (13). Pulmonary hypertension (a mean PASP
of 49 ± 7 mmHg vs PASP of 27 ± 5 mmHg) results in lower
peak longitudinal RV free wall (RVF) strain (-27.3± 7.1
% vs. -31.9 ± 8.7%, P < 0.04), longer time to peak RVF
strain (448 ±57 ms vs. 411 ±43 ms; P < 0.03) and evidence
of significant RV dyssynchrony (-83±55 ms vs. 1± 17
ms, P < 0.00001) (14). RV mechanical delay can increase
in proportion to pulmonary pressure (15). RV and left
ventricular dyssynchrony were detected by Tissue Doppler
Imaging in HF patients, but behaviors of the ventricular dyssynchron were different in the two ventricles. Mean time
of right ventricular dyssynchrony was 59 ± 45 ms, while the
mean time of the left ventricular dyssynchrony was 80 ± 62
ms. There was a strong correlation between right ventricular
dyssynchrony and pulmonary artery systolic pressure (r =
0.73; P < 0.001) and a negative correlation between right
ventricular dyssynchrony and right ventricular fractional
area change (r = -0.43; P < 0.02) (16). The increase of
right ventricular systolic pressure (RVSP) would lead to
right heart insufficiency. The baseline RVSP>35mmHg
was associated with worse clinical outcome after CRT
(17). Progressive RV dysfunction and RV failure causes
increased morbidity and mortality in patients with chronic
heart failure and elevation of pulmonary arterial pressure
(18,19,20).
Pulmonary hypertension is a ssociated with an
increased left atrial pressure. Congestive heart failure
leads pulmonary hypertension by an increased left atrium
pressure. Pulmonary hypertension either due to increased
left atrial pressure or high pulmonary vascular resistance
can lead to the worse clinic outcome in these patients (21).
The past studies have provided evidence that PASP greater
than 50 mmHg is associated with poor clinical outcomes.
(22,22,23). Stern et al found that compare when compared
to PASP <50mmHg the patients with PASP≥50mmHg had a
significantly worse survival (p=0.02), clinical outcome and
poor LV reverse remodeling after CRT (p=0.045) (21).
Our results showed though the total mortality was
higher in patients with PASP>50mmHg, but there were no
statistical different in all 3 groups of patients with PASP
either greater or less 50 mmHg. The main cause of death
was sudden cardiac death and non-cardiac diseases in
patients with PASP≤50mmHg, and decompression of heart
failure in patients with PASP>50mmHg. In patients with
PASP>50mmHg, the risk of death from decompression
of heart failure was higher than in patients with PASP≤
50mmHg (P<0.01). Based on our experience, CRT has
improved the left ventricular function and locomotivity,
and the most noted improvements of left ventricular
function and locomotivity were seen in patients with PASP
≤30mmHg. Reverse remodeling was a measurement of
LV response to CRT, while the elevated PASP did not
predict the presence of LV reverse remodeling, and patients
with PASP≤30mmHg LV reverse remodeling had a better
prognosis in terms of improvement in cardiac function and
survival benefit after CRT implantation. The LVEF was
still an important conventional marker of response of CRT,
which may be less relevant in patients with elevated PASP,
but our finding improvement of LVEF in patients with
normal PASP was very significant compared to elevated
PASP (p<0.01). To further investigate the efficacy and
clinical outcomes, large size prospective studies with longer
follow-up should be conducted.
In conclusion, elevated PASP has no prognostic effect on improvement in heart functioning in patients undergoing
CRT. However, it is associated with worse LV remodeling
and increased mortality due to decompensated heart failure.
|
|
References
Cite this article as: Wang DM, Han YL, Zang HY, Yu HB, Wang SL, Wang ZL, Jing QM. Prognostic effects of pulmonary hypertension in patients undergoing cardiac resynchronization therapy. J Thorac Dis 2010;2(2):71-75. doi: 10.3978/j.issn.2072-1439.2010.02.02.004
|