Chronic suppurative lung disease in adults
Introduction
Bronchiectasis is defined as irreversible dilatation of the bronchi (1-3). This definition has been challenged in the pediatric literature due to the prevalence of children with clinical characteristics similar to bronchiectasis, but without bronchial dilatation, leading to the term chronic suppurative lung disease (CSLD) (4-7). Emerging data suggest that early diagnosis and intensive therapy may slow the decline in lung function and lead to improved outcomes in these patients (5,8-10). A widely accepted paradigm in the pediatric literature is that a continuum of disease exists, with “pre-bronchiectasis” representing a stage of productive purulent cough, and frequent pulmonary infections without airway dilatation (5,11,12). This may ultimately lead to airway damage and dilatation that is defined as bronchiectasis (4,5,11). This type of patient is not widely recognized in adults. We present four such adult patients who were seen at the Center for Bronchiectasis Care at the University of Connecticut Health Center. Institutional review board approval was not required as this case series was not considered human subject research.
Case 1
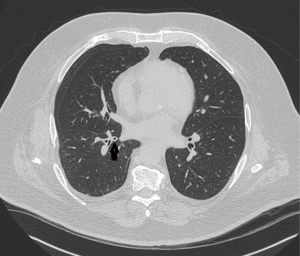
A 74-year-old man with adult-onset asthma diagnosed in his 60s was referred for a 1 year history of daily cough productive of yellow-green sputum. He was a lifelong nonsmoker. His physical examination demonstrated coarse breath sounds. Pulmonary function testing showed mild restriction, likely related to obesity. High resolution computed tomography (HRCT) only showed scattered areas of bronchial wall thickening (Figure 1). Sputum culture grew only methicillin resistant Staphylococcus aureus (MRSA). He was treated with a course of doxycycline with near compete resolution of his cough. Three months later his cough worsened and repeat sputum cultures grew Penicillium and Cladosporium species and Mycobacterium gordonae (all considered to be colonizers and therefore not treated). Immunoglobulin levels and an alpha-1-antitrypsin level were normal. He was now producing a half cup of yellow-green sputum daily, thus it was felt that he was a good candidate for azithromycin at 250 mg 3 times a week, as would be indicated for bronchiectasis patients. Three months later, he reported an “80% improvement” in his cough, which was then productive of only scant clear sputum and he has continued to do well with azithromycin and an airway clearance regimen.
Case 2
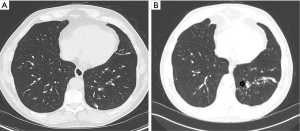
A 66-year-old male with a history of life-long asthma which was reasonably well controlled without systemic steroids, was referred for a several year history of daily cough of approximately a tablespoon of yellow sputum. His respiratory symptoms periodically worsened, and he experienced six exacerbations requiring antibiotics in the year prior to his referral. He had a 12-pack-year smoking history, discontinued more than 20 years previously. Physical examination was significant for a wet cough, rhinitis and diminished breath sounds at the lung bases. Spirometry testing showed borderline low FEV1/FVC ratio of 71% and sagging of the expiratory loop, but an FEV1 of 111% of predicted and no bronchodilator response. Blood work showed an elevated IgE level at 590 kU/L. Other immunoglobulins, alpha-1 antitrypsin level and a cystic fibrosis (CF) mutation screen were normal. Sputum cultures showed Aspergillus fumigatus, Bordetella bronchiseptica, Pseudomonas aeruginosa and Mycobacterium avium complex (MAC). Aspergillus IgG and IgE were elevated, although the IgE elevation was borderline (0.47 kU/L, upper limit of normal 0.32 kU/L), suggesting the possibility of allergic bronchopulmonary aspergillosis. An HRCT from approximately 3 years prior to his referral, but after the onset of symptoms, demonstrated some areas of bronchial wall thickening but no bronchiectasis (Figure 2A). A HRCT after his referral demonstrated bronchiectasis, bronchial wall thickening and mucus impaction in his left lower lobe that had not been present previously (Figure 2B). Oral antibiotics for the Pseudomonas and the B. bronchiseptica provided only brief improvement. A second sputum sample grew MAC. His symptoms markedly improved with therapy for MAC.
Case 3
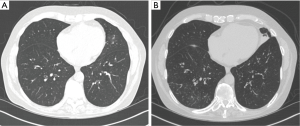
A 69-year-old white male with past medical history of asthma since childhood and multiple episodes of pneumonia was referred for evaluation. During the preceding year, he had five pneumonias, each with acute symptoms and radiographic evidence of pneumonia. Sputum cultures grew MRSA, Streptococcus pneumoniae, Hemophilus influenza and M. gordonae (M. gordonae considered to be a colonizer and therefore not treated). He was treated each time with antibiotics and clinically improved. Between the pneumonias, he reported daily cough, initially productive of about 6 to 7 teaspoons of clear sputum, which had over time progressed to become chronically discolored. He denied any childhood infections and was a lifelong nonsmoker. Physical examination demonstrated mild wheezing. Pulmonary function testing showed moderately reduced expiratory flow rates, with a significant bronchodilator response. A HRCT demonstrated diffuse airway wall thickening, but no bronchiectasis. A CF mutation screen, IgG, IgA, IgM and alpha-1-antitrypsin levels were normal. His IgE level was elevated at 527 kU/L but there was no eosinophilia. He had undetectable mannose binding lectin, a component of the innate immune system. A sputum culture grew MAC, but subsequent mycobacterial cultures were negative. He underwent bronchoscopy which revealed normal anatomy; bronchial washings grew Aspergillus flavus, Proteus vulgaris and Moraxella catarrhalis. Given his frequent pneumonias, prophylactic antibiotic therapy was implemented with daily 500 mg of cefuroxime, along with an airway clearance regimen. His recurrent pneumonias were prevented and his productive cough decreased, going from 6 to 7 teaspoons of daily sputum production to just 2 teaspoons. When his sputum production worsened approximately 3 years after his evaluation, sputum cultures revealed P. aeruginosa and Stenotrophomonas maltophilia and a repeat HRCT demonstrated disease progression, with the previously near-normal lower lung zones (Figure 3A) demonstrating marked bronchial wall thickening, bronchiectasis and tree-in-bud nodularity (Figure 3B).
Case 4
A 53-year-old female with a history of adult onset asthma diagnosed approximately 6 years previously was referred for a 6-month history of chronic cough with greenish sputum. She had experienced three exacerbations of her cough and sputum, each time treated with antibiotics with temporary improvement in her symptoms. Her physical exam was significant for rhinitis and clear lungs. An HRCT showed mild thickening of the central airways with no bronchiectasis. A sweat chloride test, immunoglobulin levels, alpha-1 antitrypsin level, and Aspergillus antibodies were normal except for borderline-low IgM levels at 46 mg/dL. Sputum cultures grew Candida albicans and Rhodotorula species. Pulmonary function testing was normal. She had a poor antibody response to vaccinations, leading to the diagnosis of functional antibody deficiency. She was started on azithromycin 250 mg 3 times a week, and an airway clearance regimen and reported feeling 75% better, with a decrease in sputum production from 1 cup per day to approximately 4 teaspoons. After starting immunoglobulin replacement for her antibody deficiency, she was trialed off of azithromycin, however within 6 weeks, she developed increased cough and sputum, prompting its reintroduction.
Discussion
We present four adults with a clinical syndrome characterized by chronic cough productive of purulent sputum and growth of various pathogens and commensals, similar to patients with bronchiectasis, but with no bronchial dilatation on HRCT initially. One patient was found to have MAC infection and may have had allergic bronchopulmonary aspergillosis. Another patient had mannose binding lectin deficiency, a condition impairing the innate immune response that may be associated with increased severity of bronchiectasis (13), but does not appear to increase the risk for bronchiectasis. Another had functional antibody deficiency. All four patients carried a diagnosis of asthma, which has been rarely associated with bronchiectasis (14), although in two, pulmonary function showed no obstruction and the asthma diagnosis was adult onset, suggesting the possibility that the symptoms attributed to asthma were actually related to CSLD. Our patients’ symptoms of chronic purulent sputum production, infection with unusual organisms and the presence of underlying immune defects are not suggestive of either neutrophilic or eosinophilic asthma.
Our patients also differed from the usual patient with chronic bronchitis. They had minimal or no tobacco or environmental dust exposure. They had chronic purulent sputum production, in contrast to patients with chronic bronchitis in whom the sputum is usually not purulent except during exacerbations (15). The organisms that they grew; Gram negatives, MRSA, various fungi and non-tuberculous mycobacteria, are less commonly seen in chronic bronchitis (16). The concept of pre-bronchiectasis has been embraced in the pediatric literature and referred to as CSLD (4-7). Our case series suggests that this phenotype is also seen in adults, although it is less well characterized. However, Schaefer et al. reported a series of patients with chronic cough some of whom were found to have suppurative airways disease when bronchoscopy was performed, but not bronchiectasis (17).
There is increasing evidence for improved outcomes with earlier diagnosis and treatment of bronchiectasis (5,8-10,18,19), and this is potentially true for the type of patient we describe. An Australian study of adults with newly diagnosed bronchiectasis showed correlation between the decline in FEV1 and the duration of cough (20). Longitudinal data in children with bronchiectasis suggest that early diagnosis and intensive treatment slows deterioration of lung function (9,10). Gharagozlou showed that in patients with primary hypogammaglobulinemia, there had been a longer delay in diagnosis among those who developed bronchiectasis compared to those who did not (21).
Our findings suggest that some patients with chronic cough and purulent sputum may represent a continuum of bronchiectasis-like disease, with not all of them having bronchial dilatation early in their course. Whether or not adult patients who present in this manner are destined to progress to bronchiectasis, it may important to recognize these patients, who seem to differ from the usual patient with chronic bronchitis. Prompt recognition is important, in order to alleviate symptoms, treat underlying conditions and potentially delay disease progression. Since these patients likely have less severe local impairment of mucosal function and mucus clearance than patients with full-blown bronchiectasis, it is attractive to consider whether a pathogen “eradication” regimen (22) for chronically colonized patients might be more successful than in bronchiectasis patients. The association with asthma in this small cohort suggests that asthma may be a contributing factor, but a larger population would need to be studied to verify this.
In summary, adults may develop CSLD or pre-bronchiectasis. Pulmonologists should be aware of this entity when evaluating patients with chronic purulent sputum and no history of exposure to inhaled irritants. Sputum cultures demonstrating bacteria commonly seen in bronchiectasis patients, fungi and non-tuberculous mycobacteria should also raise suspicion for this entity. These patients may differ significantly from the typical chronic bronchitis patient in terms of pathophysiology, treatment and prognosis. We suggest that it is appropriate to evaluate and treat patients with this clinical presentation as would be done for bronchiectasis patients, despite the lack of bronchial dilatation. If this syndrome truly represents “pre-bronchiectasis” in some patients, aggressive care might forestall or prevent progression to bronchiectasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: All patients gave written consent for their information to be included in this report, except one patient who could not be located despite exhaustive attempts to do so.
References
- Westcott JL. Bronchiectasis. Radiol Clin North Am 1991;29:1031-42. [PubMed]
- Airway diseases. In: Webb WR, Müller NL, Naidich DP. editors. High resolution CT of the lung. 3rd ed. Philadelphia: Lippincott, Williams & Wilkins, 2001:467-546.
- Naidich DP, McCauley DI, Khouri NF, et al. Computed tomography of bronchiectasis. J Comput Assist Tomogr 1982;6:437-44. [Crossref] [PubMed]
- Chang AB, Byrnes CA, Everard ML. Diagnosing and preventing chronic suppurative lung disease (CSLD) and bronchiectasis. Paediatr Respir Rev 2011;12:97-103. [Crossref] [PubMed]
- Chang AB, Redding GJ, Everard ML. Chronic wet cough: Protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr Pulmonol 2008;43:519-31. [Crossref] [PubMed]
- Nikolaizik WH, Warner JO. Aetiology of chronic suppurative lung disease. Arch Dis Child 1994;70:141-2. [Crossref] [PubMed]
- Eastham KM, Fall AJ, Mitchell L, et al. The need to redefine non-cystic fibrosis bronchiectasis in childhood. Thorax 2004;59:324-7. [Crossref] [PubMed]
- Haidopoulou K, Calder A, Jones A, et al. Bronchiectasis secondary to primary immunodeficiency in children: longitudinal changes in structure and function. Pediatr Pulmonol 2009;44:669-75. [Crossref] [PubMed]
- Bastardo CM, Sonnappa S, Stanojevic S, et al. Non-cystic fibrosis bronchiectasis in childhood: longitudinal growth and lung function. Thorax 2009;64:246-51. [Crossref] [PubMed]
- Kapur N, Masters IB, Chang AB. Longitudinal growth and lung function in pediatric non-cystic fibrosis bronchiectasis: what influences lung function stability? Chest 2010;138:158-64. [Crossref] [PubMed]
- Donnelly D, Critchlow A, Everard ML. Outcomes in children treated for persistent bacterial bronchitis. Thorax 2007;62:80-4. [Crossref] [PubMed]
- Marchant JM, Masters IB, Taylor SM, et al. Utility of signs and symptoms of chronic cough in predicting specific cause in children. Thorax 2006;61:694-8. [Crossref] [PubMed]
- Chalmers JD, McHugh BJ, Doherty C, et al. Mannose-binding lectin deficiency and disease severity in non-cystic fibrosis bronchiectasis: a prospective study. Lancet Respir Med 2013;1:224-32. [Crossref] [PubMed]
- Oguzulgen IK, Kervan F, Ozis T, et al. The impact of bronchiectasis in clinical presentation of asthma. South Med J 2007;100:468-71. [Crossref] [PubMed]
- Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196-204. [Crossref] [PubMed]
- Siempos II, Michalopoulos A, Falagas ME. Treatment of acute bacterial exacerbations of chronic bronchitis. Expert Opin Pharmacother 2009;10:1173-82. [Crossref] [PubMed]
- Schaefer OP, Irwin RS. Unsuspected bacterial suppurative disease of the airways presenting as chronic cough. Am J Med 2003;114:602-6. [Crossref] [PubMed]
- Chang AB, Masel JP, Boyce NC, et al. Non-CF bronchiectasis: clinical and HRCT evaluation. Pediatr Pulmonol 2003;35:477-83. [Crossref] [PubMed]
- Edwards EA, Metcalfe R, Milne DG, et al. Retrospective review of children presenting with non cystic fibrosis bronchiectasis: HRCT features and clinical relationships. Pediatr Pulmonol 2003;36:87-93. [Crossref] [PubMed]
- King PT, Holdsworth SR, Farmer M, et al. Phenotypes of adult bronchiectasis: onset of productive cough in childhood and adulthood. COPD 2009;6:130-6. [Crossref] [PubMed]
- Gharagozlou M, Ebrahimi FA, Farhoudi A, et al. Pulmonary complications in primary hypogammaglobulinemia: a survey by high resolution CT scan. Monaldi Arch Chest Dis 2006;65:69-74. [PubMed]
- White L, Mirrani G, Grover M, et al. Outcomes of Pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir Med 2012;106:356-60. [Crossref] [PubMed]