Efficiency and safety of roflumilast combined with long-acting bronchodilators on moderate-to-severe stable chronic obstructive pulmonary disease patients: a meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is a devastating disease which causes high morbidity and mortality worldwide (1,2). Global Burden of Disease (GBD) Study projected that COPD was estimated as the direct underlying cause of 7.8% of all deaths and will become the third leading cause of death worldwide by 2020 (3-5).
COPD exacerbations, defined as an acute worsening of a patient’s baseline symptoms (6), are accompanied by decreased quality of life (7), and further elevated risk of mortality and morbidity with each successive exacerbation compared with baseline disease (6,8-10). Severe COPD is associated with periodic exacerbations of respiratory symptoms that need aggressive treatment and often necessitate hospital admission. These exacerbations worsen patient health status, accelerate decline in lung function, and increase mortality. It is reported that many COPD patients still suffer from exacerbations after treated with maximum recommended therapy that consists of a combined therapy: inhaled corticosteroids (ICS), long-acting β-agonist (LABA) and long-acting muscarinic antagonist (LAMA). In addition, it is not a good treatment to increase the doses of long-acting bronchodilators (LABA and/or LAMA) and ICS due to the flat dose-response curve and the increasing risk of side effects, especially pneumonia associated with ICS (11,12) and stroke with inhaled SABA (13).
Roflumilast is an oral phosphodiesterase-4 inhibitor with anti-inflammatory actions both in vitro and in vivo. It consistently improves lung function and reduces the frequency of exacerbations in patients with severe COPD, symptoms of chronic bronchitis, and a history of frequent exacerbations (6). Roflumilast inhibits the hydrolysis of cyclic adenosine monophosphate (CAMP) in inflammatory cells (14). The clinical studies showed it could reduce exacerbations in patients with COPD (15-18). The efficacy and safety of roflumilast were evaluated in several reviews and meta-analysis (5,19-21), however, the efficacy or safety of roflumilast combined with long-acting bronchodilators (LABA and/or LAMA) was not independently evaluated. Therefore, we conducted meta-analysis to investigate the efficacy and safety of roflumilast combined with long-acting bronchodilators in COPD patients.
Methods
Search strategy and eligibility criteria
This meta-analysis was conducted according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (22). We searched from PubMed, Embase, Google Scholar and the Cochrane Central Register of Controlled Trials at the beginning of February 5th 2016 to identify relevant RCTs. The following search terms were used without any restrictions: ‘‘Phosphodiesterase Inhibitors’’ or “PDE4” or “roflumilast”, ‘‘chronic obstructive pulmonary disease’’ or ‘‘COPD’’. In addition, in order to identify these studies that were not identified from the preliminary literature searches, reference lists of retrieved articles were also reviewed.
Eligible clinical trials were defined based on the following criteria: (I) population: eligible patients ≥40 years old; current or former smokers (≥1 year of smoking cessation) with more-than-10-years’ smoking history. As diagnosed with COPD, they had the airflow limitation which was changed from moderate to very severe (verified by forced expiratory volume in 1 s of the post-bronchodilator or the forced vital capacity ratio (FEV1/FEC)<0.70 and FEV1pred <80%); (II) intervention: the patients in the experimental group have received 500 µg roflumilast combined with long-acting bronchodilators; (III) control: the patients in the control group have received placebo and combined with long-acting bronchodilators; (IV) outcomes: the COPD exacerbations and adverse events were the outcomes as measured; (V) type of research: the research should be randomized controlled trials (RCTs).
Articles could not be included if from the meta-analysis and based on the following standards: (I) letters, papers from conference, comments, and review articles; (II) studies with stacking or duplicate data; (III) Patients were diagnosed with other relevant lung diseases, lower respiratory tract infection, diagnosis of asthma at <40 years old, or α1-antitrypsin deficiency.
Quality assessment
The bias risk of the studies was independently scored by two investigators using the Cochrane risk-of-bias tool (23). A low value, unclear or high risk bias was assigned in the following domains: generation in random sequence generation, allocation concealment of allocation, blinding of participants and personnel, blinding of outcome assessment, incomplete data of the outcome data, selective reporting and other biases. When the consensus was concluded, all the disagreements could be resolved. Trials were considered to be at high risk of bias if having high bias risk for any one or more key domains. On the contrary, trials were considered to be at low risk in bias if having low risk in bias for all key domains. Otherwise, trials were considered to have an unclear risk of bias (23).
Data extraction
Two researchers evaluated and extracted the data independently. All studies were checked for twice. Moreover, the disagreements were resolved by consensus. The data elements extracted in this review included the following factors: (I) the details of publication, including the first author’s last name, year of publication; (II) characteristics of the studied population, including the sample size, age, and stage of disease; (III) the data about COPD exacerbations and other adverse events.
Statistical analysis
For dichotomous outcomes, the differences were calculated using relative risks (RRs). With the relevant 95% confidence intervals (CIs) were estimated in the study. The model of fixed-effects (24) was adopted in the study. Overall, the heterogeneity was tested by using the I2 statistic. If I2 >50% or P<0.1, we investigated different effects through subgroup analysis according to the application of roflumilast combined long-acting bronchodilators or ICS. All statistical analysis was carried out by using RevMan 5.1.0 (The Cochrane Collaboration, Oxford, UK).
Results
Search and study selection
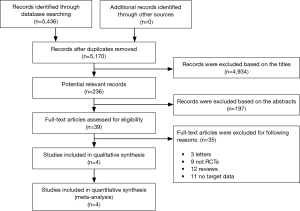
At the beginning of the research, we authenticated total 5,436 records. After retrieving the titles and abstracts, 4,934 studies were excluded based on the titles, 197 studies were excluded based on the abstracts, 35 studies (3 letters, 9 observational studies, 12 reviews and 11 studies without target data) were excluded through article types. According to the meta-analysis, six RCTs (15-18) were included according to the inclusion standard. Moreover, two separate trials were also included in two studies [Fabbri et al. (17)and Calverley et al. (18)]. The flow diagram of the study represented the reasons for exclusion and reasons of studies included were shown in the Figure 1.
Study characteristics
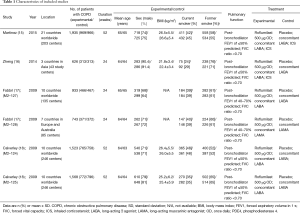
The population of the trials ranged from 626 to 1,935, with total 7,329 patients including 3,670 in the experimental group and 3,659 in the control group. The detail characteristics were shown in Table 1.
Full table
Risk of bias of studies
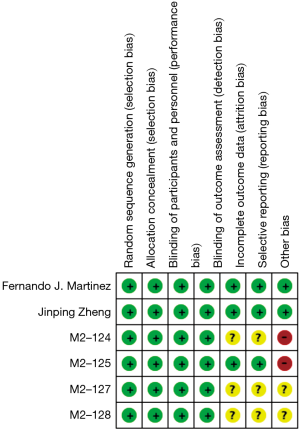
Two trials (15,16) were judged to be in low risk in bias; two trials (18) were judged to be in unclear risk of bias, and two trials (17) were judged to be in high risk of bias as shown in the Figure 2.
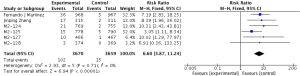
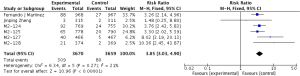
Primary outcome: COPD exacerbations
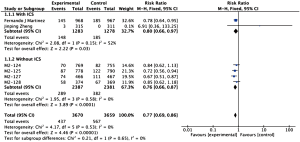
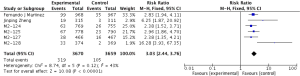
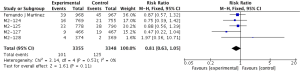
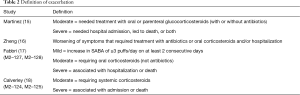
The definitions of exacerbation varied among various studies and were summarized in Table 2. Data of exacerbations were available in all 4 trials (15-18). The overall exacerbation data in experimental and control groups were 437 (11.9%) of 3,670 in the experiment and 567 (15.5%) of 3,659 control groups respectively. Overall, in the experimental group, the exacerbations of the fixed-effects model was significantly reduced in the experimental group (RR, 0.77; 95% CI, 0.69 to 0.86; P=0.53; I2=0%). According to the subgroup analysis, the difference was not significant between roflumilast combined with long-acting bronchodilators (RR, 0.76; 95% CI, 0.66 to 0.87; P=0.58; I2=0%) and that with ICS (RR, 0.8; 95% CI, 0.66 to 0.97; P=0.15; I2=52%). It revealed there was no significance (P=0.65), as shown in the Figure 3.
Full table
Secondary outcomes: safety
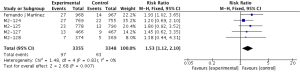
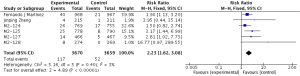
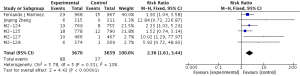
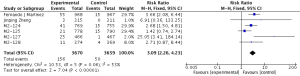
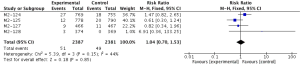
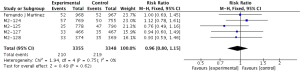
Compared to placebo combined with long-acting bronchodilators, roflumilast with long-acting bronchodilators caused severe back pain (RR 1.53; 95% CI 1.12–2.10), diarrhea (RR 3.03; 95% CI 2.44–3.76), headache (RR 2.23; 95% CI 1.62–3.08), insomnia (RR 2.36; 95% CI 1.61–3.44), nausea (RR 3.09; 95% CI 2.26–4.23) and significantly decreased appetite (RR 6.60; 95% CI 3.87–11.24) and weight (RR 3.35; 95% CI 3.03–4.90). Roflumilast could not significantly increase the incidences of influenza (RR 1.04; 95% CI 0.70–1.53), nasopharyngitis (RR 0.96; 95% CI 0.80–1.15), or respiratory tract infection (RR 0.63; 95% CI 0.63–1.05), as shown in Figures S1-S10.
Discussion
The data was carried out to form four articles (including six RCTs) to evaluate the effect and safety of roflumilast combined with long-acting bronchodilators in COPD patients. It showed that roflumilast combined with long-acting bronchodilators could lead reduction in exacerbations to moderate-to-severe COPD patients and cause some side effects.
Severe COPD is associated with periodic exacerbations of respiratory symptoms that requires aggressive treatment and often necessitates hospital admission (25,26). The treatment with long-acting inhabited bronchodilators, with or without ICS, may possibly cause the periodic exacerbations of respiratory symptoms. The phosphodiesterase-4 inhibitors could reduce the rate of exacerbations and hospital admission (25). Recently, several studies about meta-analysis have only discussed the relationship between roflumilast and COPD exacerbations. According to the Cochrane review (19) of 15 studies on 12,654 patients, it was found that there was a statistically significant difference between 500 µg roflumilast 500 µg once daily (OD) and placebo in exacerbation rate and pre-bronchodilator FEV1. Nevertheless, the effect and safety were not explored under the condition of concomitant treatment with long-acting bronchodilators, which was a recommended pharmacological treatment to prevent exacerbations of stable COPD.
The previous studies suggested that roflumilast could lead to significant reduction in frequent exacerbations in severe COPD patients when being added to ICS and long-acting bronchodilators (15,27). Besides, Roflumilast combined with a LABA with or without an ICS or a LAMA appeared to be a reasonable alternative choice for patients with severe-to-very-severe COPD (6), however, according to the guideline released by GOLD (25), phosphodiesterase-4 inhibitors should always be used together with at least one long-acting bronchodilator and there were still few studies to determine whether the combination of long-acting bronchodilators is more effective than the exclusive use of one to prevent the disease from being exacerbated. So our meta-analysis may provide more evidences for the guideline released by GOLD.
In our meta-analysis, two RCTs (15,16) were combined with ICS and long-acting bronchodilators, but the additional effect of ICS was unknown in reducing exacerbations. So we performed a subgroup analysis of roflumilast combined with or without ICS and long-acting bronchodilators. The result revealed that both groups could lead to reduction in exacerbations, but the significantly difference was not detected.
In terms of safety, when roflumilast was added, the side effects were more common and could lead to discontinuation of therapy (27). The result showed that roflumilast combined with long-acting bronchodilators was associated with some adverse events (such as back pain, diarrhea, nausea, weight loss and decreased appetite). The previous studies (19-21) have clearly shown that gastrointestinal disturbances including nausea and diarrhea were more significant in the experimental group than of the placebo group. Furthermore, besides the psychiatric symptoms such as headache and insomnia, our study showed that roflumilast could cause significant back pain noticeably compared with placebo. If the patients received roflumilast therapy, they would suffer from decreased appetite and weight loss. However, the detailed reason was still unknown. In addition, a lot of studies had reported nasopharyngitis, influenza, and respiratory tract infection and other side effects but there was still no sufficient evidence to show that roflumilast caused these side effects. Moreover, the infectious incidents may be related to the use of ICS. Its effect has been confirmed to be associated with the infection (11,12). It might explain there was no statistical difference between the experimental and control groups in nasopharyngitis, influenza, and respiratory tract infection. It also could be the agent that the ICS group may cause the withdrawal from the study and lead to large heterogeneity of the subgroup due to some infectious factors according to the subgroup analysis.
There were some limitations in our study. Firstly, in subgroup analysis, the heterogeneity of the ICS group was quite large. Hence, the subgroup analysis could not draw the definite conclusions and more evidences were required to be revealed under the circumstance of roflumilast combined with ICS and long-acting bronchodilators. Secondly, the data of lung function could not be combined due to the differences in lung function parameters among the six RCTs. Consequently, we did not evaluate the commonly used indices such as FEV1 and FVC. Further studies should be focused on the improvements in lung function parameters after treatment with roflumilast combined with long-acting bronchodilators. Last but not least, rare adverse events such as trail fibrillation and suicide were more significantly commonly associated with roflumilast. However such events may happen just by accident (20). Since the adverse events were related to roflumilast combined with long-acting bronchodilators, further studies were needed to focus on the long-term efficacy and safety of roflumilast as well as findings in universal COPD patients.
Conclusions
Roflumilast combined with long-acting bronchodilators is a better option for moderate-to-severe COPD patients than exclusive use of long-acting bronchodilators in reducing exacerbations. However, it can cause some side effects. Further studies need consider well enough of the benefits and adverse events caused by roflumilast combined with long-acting bronchodilators.
Acknowledgements
Funding: This work was supported by the Guangdong Provincial Science and Technology Project (2013B022000072; 201507020033) and Guangzhou Municipal Science and Technology Project (201507020033).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412. [Crossref] [PubMed]
- Choi SM, Lee J, Park YS, et al. Prevalence and global initiative for chronic obstructive lung disease group distribution of chronic obstructive pulmonary disease detected by preoperative pulmonary function test. PLoS One 2015;10:e0115787. [Crossref] [PubMed]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [Crossref] [PubMed]
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163-96. [Crossref] [PubMed]
- Luo J, Wang K, Liu D, et al. Can roflumilast, a phosphodiesterase-4 inhibitor, improve clinical outcomes in patients with moderate-to-severe chronic obstructive pulmonary disease? A meta-analysis. Respir Res 2016;17:18. [Crossref] [PubMed]
- Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis 2016;11:81-90. [Crossref] [PubMed]
- Seemungal TA, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22. [Crossref] [PubMed]
- Seneff MG, Wagner DP, Wagner RP, et al. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA 1995;274:1852-7. [Crossref] [PubMed]
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31. [Crossref] [PubMed]
- Suissa S, Dell'Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012;67:957-63. [Crossref] [PubMed]
- Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med 2013;1:210-23. [Crossref] [PubMed]
- Zervas E, Samitas K, Gaga M, et al. Inhaled corticosteroids in COPD: pros and cons. Curr Drug Targets 2013;14:192-224. [Crossref] [PubMed]
- Lin HW, Chung CL, Lin YS, et al. Inhaled Pharmacotherapy and Stroke Risk in Patients with Chronic Obstructive Pulmonary Disease: A Nationwide Population Based Study Using Two-Stage Approach. PLoS One 2015;10:e0130102. [Crossref] [PubMed]
- Sanz MJ, Cortijo J, Morcillo EJ. PDE4 inhibitors as new anti-inflammatory drugs: effects on cell trafficking and cell adhesion molecules expression. Pharmacol Ther 2005;106:269-97. [Crossref] [PubMed]
- Martinez FJ, Calverley PM, Goehring UM, et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015;385:857-66. [Crossref] [PubMed]
- Zheng J, Yang J, Zhou X, et al. Roflumilast for the treatment of COPD in an Asian population: a randomized, double-blind, parallel-group study. Chest 2014;145:44-52. [Crossref] [PubMed]
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009;374:695-703. [Crossref] [PubMed]
- Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685-94. [Crossref] [PubMed]
- Chong J, Poole P, Leung B, et al. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011.CD002309. [PubMed]
- Oba Y, Lone NA. Efficacy and safety of roflumilast in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ther Adv Respir Dis 2013;7:13-24. [Crossref] [PubMed]
- Yan JH, Gu WJ, Pan L. Efficacy and safety of roflumilast in patients with stable chronic obstructive pulmonary disease: a meta-analysis. Pulm Pharmacol Ther 2014;27:83-9. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65. [Crossref] [PubMed]
- O'Reilly J, Jones MM, Parnham J, et al. Management of stable chronic obstructive pulmonary disease in primary and secondary care: summary of updated NICE guidance. BMJ 2010;340:c3134. [Crossref] [PubMed]
- Muñoz-Esquerre M, Diez-Ferrer M, Montón C, et al. Roflumilast added to triple therapy in patients with severe COPD: a real life study. Pulm Pharmacol Ther 2015;30:16-21. [Crossref] [PubMed]