Region specific lung nodule management practice guideline
Following the recommendation by the United States Preventive Services Task Force (USPSTF) and the final decision on February 5, 2015 by the Centers of Medicare and Medicaid Services (CMS) to cover computed tomography (CT) screening of lung cancer (1-3), CT screening has begun in the United States. Other countries, such as Canada, are planning to implement population based CT screening programs. A large number of lung nodules will be found in addition to incidental lung nodules from diagnostic CTs such as CT coronary angiogram or abdominal CTs. In the US, it was estimated that in an adult population of over 234 million, >1.5 million Americans will be found to have a lung nodule (4). Only ~5% of them will be found to have lung cancer (4). This means a large proportion of patients with lung nodules will have repeat CT imaging studies, PET scans, biopsies or surgery for benign disease with implication in health care resource utilization. For individuals with benign nodules, surveillance provides no benefit, may delay diagnosis of infectious granulomas, incur costs for physician/imaging study visits or suffer from harm of invasive procedures and ionizing radiation. A significant proportion of patients with incidental pulmonary nodules were found to experience clinically significant distress (5). Therefore, it is important to have evidence-based guidelines for timely efficient management of lung nodules—whether screen detected or incidental.
Several professional societies have published guidelines on management of lung nodules such as the Fleischner Society (6,7), the American College of Chest Physicians (ACCP) (8), the National Comprehensive Cancer Network (NCCN) (9), the British Thoracic Society (BTS) (10,11) and the American College of Radiology (12). The European NELSON trial has also published their protocol for management of screen detected lung nodules using volumetric analysis (13,14). These guidelines/protocols differ in the frequency and duration of repeat CT imaging studies, use of PET and the threshold for referral for tissue diagnosis or treatment (Table 1) with implication in health care resource utilization and costs as well as potential harms to patients. The major differences in guidelines are related to patient risk assessment and nodule type and size. The recently published clinical practice consensus guidelines for evaluation of pulmonary nodules for patients in Asia (15) have raised important questions in lung nodule management in different regions of the world with different exposures such as outdoor and indoor pollution in addition to tobacco smoking, genetic susceptibility (16) and prevalence of granulomas from infections such as tuberculosis.
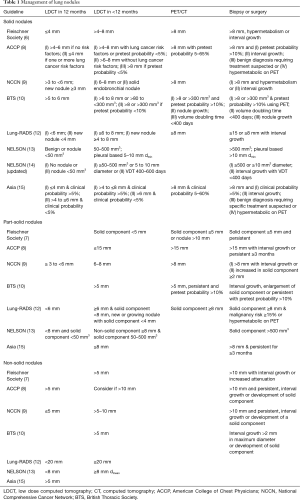
Full table
Currently, there are at least 20 lung cancer risk prediction models (17,18). The Tammemagi PLCOm2012 and PLCOall2014 models, the Katki model (both based on the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial and the National Lung Screening Trial (17-20) and the Hoggart European Prospective Investigation into Cancer and Nutrition (EPIC) model (21) are the only models that are based on large prospectively followed population-based samples not limited to people at high risk of lung cancer. These models show high discrimination and calibration in ever smokers. However, these models have ≤3% Asians. Their utility in Asian countries has not been validated. Although outdoor and household air pollution account for an estimated 29% of lung cancer deaths worldwide (22-25) and is a major problem in Asian countries, none of the lung cancer risk prediction models published so far have included outdoor and/or household pollution as one of the risk variables. Lung cancer risk prediction model that evaluate and consider these factors need to be developed and validated in Asian patients to define lung cancer risk more accurately.
Due to a much higher prevalence of granuloma from tuberculosis in many Asian countries, higher outdoor and indoor pollution exposures, high incidence of adenocarcinoma in female non-smokers and anecdotal evidence of the emergence of malignancy in stable nodules after many years of stability, the proposed Asian guideline (15) differs from the ACCP guideline (8) by incorporating clinical judgement of lung cancer risk, lesser reliance on PET imaging, longer surveillance of nodules (≥3 years even for solid nodules) and greater use of non-surgical biopsy for diagnosis. As discussed above, there is no validated lung cancer risk prediction model that has included air pollution exposures or genetic profile as risk variables. Physicians’ perception of lung cancer risk may vary. Individual lung cancer risk is not the same as lung nodule malignancy risk although some of the lung nodule malignancy risk prediction tools such as the Pan-Canadian lung nodule malignancy risk calculator take into account age, sex, family history of lung cancer and emphysema in addition to nodule type, size, spiculation and location (26). The accuracy of the PanCan model has been validated in other non-Asian countries (27,28). However, the utility of the PanCan model needs to be validated in Asia. An integrated model such as the PanCan model has advantages over those recommended in current guidelines as it incorporates nodule type in the risk assessment and action thresholds for further investigation. It is difficult to remember different size and follow-up criteria for solid, part-solid and non-solid nodules. Observer variations in nodule size measurement can affect nodule classification and management especially with manual measurement of smaller nodules (29). Repeat CT scanning introduces yet another variable with different size criteria for new nodules (9,12) and different interval growth criteria (Table 2).

Full table
The Asia Guideline (15) points to knowledge gaps in lung nodule management that needs to be addressed in future studies such as a better model of lung cancer risk that includes outdoor and indoor pollution exposures as well as genetic susceptibility. Further research is also needed in lung nodule characterization especially for nodules ≤10 mm as the likelihood of cancer is not the same as likelihood of biologically aggressive cancer that grows rapidly or with high metastatic potential. Rapid non-invasive diagnosis of infectious granuloma is also needed to facilitate treatment.
Acknowledgements
This work was supported by the Terry Fox Research Institute and the British Columbia Cancer Foundation.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Yan Xu (Department of Respiratory Medicine, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Moyer VA. U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- Final recommendation statement: lung cancer: screening. U.S. Preventive Services Task Force. Available online: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening
- Final national coverage determination on screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). Available online: http://www.cms.gov/medicare-coverage-database/details/nca-details.aspx?NCAId=274&NcaName=Screening+for+Lung+Cancer+with+Low+Dose+Computed+Tomography+(LDCT)
- Simmons J, Gould MK, Iaccarino J, et al. Systems-Level Resources for Pulmonary Nodule Evaluation in the United States: A National Survey. Am J Respir Crit Care Med 2016;193:1063-5. [Crossref] [PubMed]
- Freiman MR, Clark JA, Slatore CG, et al. Patients' Knowledge, Beliefs, and Distress Associated with Detection and Evaluation of Incidental Pulmonary Nodules for Cancer: Results from a Multicenter Survey. J Thorac Oncol 2016;11:700-8. [Crossref] [PubMed]
- MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin 2015;25:185-97. [Crossref] [PubMed]
- Baldwin DR, Callister ME. Guideline Development Group. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794-8. [Crossref] [PubMed]
- Baldwin DR, Callister ME, Graham R, et al. Pulmonary nodules again? The 2015 British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Clin Radiol 2016;71:18-22. [Crossref] [PubMed]
- Lung CT Screening Reporting and Data System (Lung-RADS™). American College of Radiology. Available online: http://www.acr.org/Quality-Safety/Resources/LungRADS
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Horeweg N, van Rosmalen J, Heuvelmans MA, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol 2014;15:1332-41. [Crossref] [PubMed]
- Bai C, Choi CM, Chu CM, et al. Evaluation of pulmonary nodules: clinical practice consensus guidelines for Asia. Chest 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Li H, Yang L, Zhao X, et al. Prediction of lung cancer risk in a Chinese population using a multifactorial genetic model. BMC Med Genet 2012;13:118. [Crossref] [PubMed]
- Tammemägi MC. Application of risk prediction models to lung cancer screening: a review. J Thorac Imaging 2015;30:88-100. [Crossref] [PubMed]
- Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728-36. [Crossref] [PubMed]
- Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med 2014;11:e1001764. [Crossref] [PubMed]
- Katki HA, Kovalchik SA, Berg CD, et al. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA 2016;315:2300-11. [Crossref] [PubMed]
- Hoggart C, Brennan P, Tjonneland A, et al. A risk model for lung cancer incidence. Cancer Prev Res (Phila) 2012;5:834-46. [Crossref] [PubMed]
- Straif K, Cohen A, Samet J. editors. Air Pollution and Cancer. Lyon, France: International Agency for Research on Cancer, IARC Scientific Publication No. 161, 2013.
- Loomis D, Grosse Y, Lauby-Secretan B, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol 2013;14:1262-3. [Crossref] [PubMed]
- Zhao Y, Wang S, Aunan K, et al. Air pollution and lung cancer risks in China--a meta-analysis. Sci Total Environ 2006;366:500-13. [Crossref] [PubMed]
- Hystad P, Demers PA, Johnson KC, et al. Long-term residential exposure to air pollution and lung cancer risk. Epidemiology 2013;24:762-72. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Winkler Wille MM, van Riel SJ, Saghir Z, et al. Predictive Accuracy of the PanCan Lung Cancer Risk Prediction Model -External Validation based on CT from the Danish Lung Cancer Screening Trial. Eur Radiol 2015;25:3093-9. [Crossref] [PubMed]
- Al-Ameri A, Malhotra P, Thygesen H, et al. Risk of malignancy in pulmonary nodules: A validation study of four prediction models. Lung Cancer 2015;89:27-30. [Crossref] [PubMed]
- van Riel SJ, Sánchez CI, Bankier AA, et al. Observer Variability for Classification of Pulmonary Nodules on Low-Dose CT Images and Its Effect on Nodule Management. Radiology 2015;277:863-71. [Crossref] [PubMed]


