Ultrasound-guided central vascular interventions, comments on the European Federation of Societies for Ultrasound in Medicine and Biology guidelines on interventional ultrasound
Introduction
Sonographic imaging of potential target vessels to determine the most appropriate vessel, the ideal puncture site and the best patient position, is a reasonable approach to identify anatomical variations known to occur in a substantial portion of veins (1-5).
Ultrasound (US) guided catheter placement into the subclavian and internal jugular veins (IJVs) was first described in 1975 (6,7). The first attempts to use a Doppler-controlled needle director as an aid for percutaneous angiography were reported in 1973. More recently US guidance for vascular access has been introduced more widely also as quality parameter to minimize complications (8). Real time ultrasound (RTUS) has proven beneficial in guiding interventional procedures under many circumstances, becoming standard in clinical practice for many years (9). Through technical advances and improvements of image quality, RTUS allows identification of vessel localisation the best target vessel and optimised puncture site (10). Anatomical variations can be easily identified (1-5) and vein thrombosis excluded which is not only of importance in oncological patients (11,12). It is important to exclude vein catheter associated thrombosis in, for example, critical care patients.
The aim of this paper is to summarize and comment on the recently published European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) on interventional ultrasound (INVUS), part VI, US-guided vascular interventions (13), to give practical advice and to illustrate the procedures. We refer also to the current EFSUMB guidelines on INVUS (13-18) on contrast enhanced ultrasound (CEUS) (19-21), elastography (22,23), and comments on the guidelines (24-29).
Basic principles
The three cornerstones for US guided vascular interventions are the patient, the interventionalist and US equipment.
Indications and contraindications
Most importantly the right indication for each kind of vascular access should be justified; this is especially true for central vascular access. The information provided to the patient depends on the situation (emergency) and on their level of consciousness (13).
Establishing central venous access is fundamental for emergency physicians in order to monitor the hemodynamics, central venous pressure (CVP) and pulse contour cardiac output (PiCCO), to deliver vasoactive drugs, hyperosmolar fluids and volume resuscitation (30). In oncological and haematological patients, central venous access is often required for blood sampling and for peripheral stem cell preparation, as well as for administration of blood products, chemotherapy or other drugs (11). Advantages of US-guidance for central venous access have been proved in a variate patient population, including critically ill patients (31,32), ventilated patients (33), both oncological and haematological patients (11,34-37), in situations when a parental nutrition was needed (37) or in haemodialised patients (38,39). Outcomes are improved for experienced as well as inexperienced operators (40). Given a clear indication, there is no absolute contraindication for US-guided vascular access and interventions.
Which central venous access?
Due to coagulation disorders, thrombocytopenia (disease- or therapy-associated) and hemostasis disorders, oncological and hematological patients belong to the high-risk group for central venous access (11,41). Additionally, anatomical changes may be encountered in patients with the primary tumor, metastases or lymphoma in the puncture region (11).
One of the advantages of the subclavian/axillary approach is that it can be used for central venous catheter placement in patients with severe burns on the face, neck, and/or proximal shoulders (42). Still, there are drawbacks related to the smaller diameter and deeper location of subclavian and axillary veins (43).
Risks and complications
The complication rates using the traditional landmark technique range from 0.3% to 18.8%, depending on multiple factors, such as patient population, site of insertion, time taken, number of needle passes and the specific definition of complications used (44-48). Evidence from meta-analyses of RCTs shows that RTUS-guided access to the IJV and subclavian vein (SV) in adults has a significantly lower failure rate as compared to the traditional ‘blind’ access and that it is associated with a decreased rate of complications, requires a shorter access time and fewer attempts for successful access (30,38,39,49-52). In the meta-analysis of Hind et al., commissioned by the British National Institute for Clinical Excellence (NICE), the relative risk of complications, of failed attempts, and failed first attempt were reduced by 57%, 86%, and 41%, respectively (49). The two most important improvements associated with US guided technique versus landmark technique are lower risks of inadvertent arterial puncture and of local hematoma (37). It is important to recognise however that adverse events may occur also under RTUS-guidance. In particular, improper catheter placement, arterial puncture, hematoma at the puncture place, air embolism, or nerve lesions have been reported (53,54). Pneumo- and/or hemothorax are very rare events if central venous puncture is performed under RTUS-guidance (50,51). Furthermore, catheter misplacement or pneumo-/hemothorax in most cases are recognized by US at the time of intervention (54-63). Thrombosis, arteriovenous fistula and pseudoaneurysms represent possible mid-/long-term complications of central venous catheter placement and all can be easily detected by means of US (64-70). In the study of Kaye et al. (n=325 patients undergoing cardiovascular surgery), complication rates after central vein catheterization (including carotid artery puncture and pneumothorax) were significantly higher for the group who received catheter placement without US-guidance, as compared to the group having catheter placement with US (71). Using US-guidance for central venous catheter placement, Cavanna et al. reported symptomatic deep-vein thrombosis of the upper limbs in 2.4% of the cases and catheter related infections in 10% of the catheters inserted. Removal of the catheter due to complications was necessary only in 2.9% of cases. No major bleeding, nerve puncture or pneumothorax was reported (34).
Despite US-guidance, posterior vessel wall puncture may occur as a complication of venous catheterisation (72,73). Factors influencing the risk of posterior wall penetration are the particular access technique (transverse vs. longitudinal approach), the speed of needle insertion, the distance between needle entry and transducer, and the angle of insertion (74).
Tips and tricks (how to avoid risks and complications)
Here are some important points to avoid unsuccesful punctures:
- Check the equipment and its function during preparation;
- Optimise the B-mode picture of the target vessel;
- Optimise positioning of the patient (e.g., Trendelenburg position), of the examiner and of the US device relative to the puncture site (aim for a comfortable working environment for the interventionalist);
- Choose the most appropriate head position in order to locate the target vein laterally rather than anterior to the artery;
- Skills training on appropriate phantoms and in normal patient conditions prior to emergency situations;
- In hypovolemic patients: give intravenous fluid before puncture;
- The indication for central lines must be well considered—sometimes peripheral vascular access meets the needs of the condition.
Patient informed consent
Each procedure intended for diagnosis or treatment must be undertaken only after informed consent has been obtained from the conscious patient (75) or legal representative, after receiving comprehensible and understandable information about the procedure’s goal and benefits, potential risks, alternatives and complications (76). There is no legal requirement for consent to be written, or be in a particular setting, however, a signed written consent form provides documentary evidence. Consent may be withdrawn at any time, even after the form has been signed, and should lead to immediate discontinuation of a procedure. It is the responsibility of the doctor to be aware of the valid legislation and ethical guidelines in their region. The European Society for Cardiovascular and Interventional Radiology and the Society of Interventional Radiology provide information on many interventional radiology procedures on their website (www.cirse.org). The Royal College of Radiologists (UK) and the British Society of Interventional Radiology has similar information at www.rcr.org.
Interventionalist
Adequate teaching, education and training are necessary for a successful procedure. The degree of US experience significantly influences complication rates (71,77). Several studies have shown that simulation-based learning of US-guided central venous access increases skills in simulated central venous catheter insertion and is more effective than traditional bedside teaching (78-81). Moreover, a recent meta-analysis of 20 comparative studies gave proof of significant improvement in performance not only at simulators but also in some clinical outcome parameters, in particular number of needle passes to achieve central venous access and frequency of pneumothorax (82). Comparable results were reported in a meta-analysis of prospective comparative cohort-studies (83). Therefore, simulation training should be included in training programs for RTUS-guided central venous access to improve the real clinical performance of trainees.
Which US equipment?
The US equipment should allow good to excellent near field resolution. Particular presets for e.g., cervical, brachial and femoral vessels are helpful.
Which transducer?
High frequency (5–17 MHz, in practice 7–12 MHz) linear transducers with a relatively small aperture of less than 4–6 cm are recommended for superficial locations. In deeper locations (e.g., femoral vessels), particularly in obese or oedematous patients, the use of a curved array abdominal probe may be necessary.
Transducer guides
Transducers may offer vendor-dependent needle guides but only a limited number of transducers are useful and most punctures will be done free hand.
Hygiene
Sterile covers
For vascular access under US-guidance, after probe decontamination, a sterile barrier is needed, which must cover both the transducer and the cable (43). Sterile covers are mandatory according to hygiene recommendations and to avoid contact of the transducer membrane with alcohol or other disinfection fluids. It is generally required to use sterile, disposable probe covers made of latex-free material and applied under aseptic conditions, following manufacturers’ instructions (84) (Figure 1). Random testing of the batch may be done in order to assess package integrity (85). If no sterile transducer covers are available, a sterile glove may be used. In a similar fashion contact gel will be placed inside, and the flat palm surface of the glove will be used to cover the scanning surface of the transducer. Attention must be paid to eliminate any air bubbles possibly interposed between the scanning surface of the transducer and the cover or glove (43).
Sterile US gel
Only sterile US gel should be used in interventional procedures, packed in small packages matching the gel requirement for one examination and a new sachet should be used for each patient (86-94). Residual product should not be used on further patients since it may be a potential vehicle for nosocomial infections. Disposable probe covers filled with sterile gel are also available (84,86-89,91,93-95). Alternatively, disinfectant solutions may be used to ensure acoustical coupling between the skin surface and the covered transducer.
Transducer decontamination
Sterile transducer covers do not eliminate the need for transducer decontamination (96-98). Sterilization of the transducer after use is necessary in procedures with a high risk of contamination. The cleaning technique of transducers using disinfection varies between manufacturers. For more details see the EFSUMB guidelines on interventional procedures (13-18,24,99).
US guiding techniques
Definition
US-guidance for venous cannulation can be performed using different approaches. Therefore, some definitions will be discussed in the following paragraphs including “landmark technique”, “direct” and “indirect” methods, US-assistance and US-guidance, free hand technique, puncture transducers and transducer mounted devices. The “direct” technique implies needle placement under permanent real-time RTUS control (US-guidance). The needle is visualized on the US monitor as an echogenic line with ring-down artefact and the cannulation process can be monitored completely by US (43). “Indirect” (or static) techniques (US-assistance) imply that US is used to locate the appropriate target vessel, to examine its topographical relations to surrounding structures and to assess its dimensions and depth from the skin. This is therefore a simplified technique, with the advantage that sterile covers are not necessary for the transducer and there is less equipment to manipulate during the sterile line insertion. Optionally, marking might be drawn or placed on the skin corresponding to the vessel’s position just at the point where the center of the transducer overlies the center of the vessel (43). Another method which has proved to be beneficial, especially for inexperienced operators, is the mechanical US-guided approach. This implies the use of an attachment to the transducer which provides a fixed needle trajectory. The method has better success rate, improved venous access time, improved average number of attempts to success and was associated with fewer complications when compared to the traditional landmark approach (43,100). Doppler US can also be used to facilitate vessel visualization.
Comparison of access techniques
Review of the literature
US-assistance vs. landmark approach
Two randomized control trials (RCTs) have demonstrated that with US-assistance (“static ultrasound” for pre-procedural evaluation) IJV catheterisation can be performed quicker in comparison to the traditional landmark technique (101,102). Furthermore first attempt success rate was higher with US assistance (101). In one RCT comparing landmark and US-assisted techniques in ventilated patients with respiratory jugular venodilation, results of cannulation did not differ with respect to first attempt cannulation, overall success rate or the incidence of arterial puncture. However, in the patients without respiratory jugular venodilation, those outcome parameters were significantly improved in the US-assisted group (33).
A further RCT comparing complications and failures of SV catheterization using the standard landmark technique and US-assisted technique found no significant differences between the two methods (45). There are no data comparing US-assistance and landmark technique for femoral venous (FV) access (13).
US-guidance versus landmark approach
US-guidance versus landmark approach has been discussed in detail in the EFSUMB guidelines (13). There is convincing evidence from meta-analyses of RCTs that RTUS-guided access to the IJV and SV in adult patients is associated with a significantly lower failure rate both overall and on the first attempt, a shorter access time, and decreased rates of arterial puncture and hematoma formation compared to the traditional anatomical landmark approach (30,38,39,49-51,103,104). These advantages were shown for particular patient groups and clinical situations, e.g., for adults requiring emergent central venous catheter placement (51,52), ventilated patients (33), critical care patients (31,32), in oncological and haematological patients (34-36), in elective situations for parenteral nutrition (37), and for placement of hemodialysis catheters (13,38,39).
US-assistance versus US-guidance
The results of RCTs comparing US-assistance and RTUS-guidance for central venous access are conflicting (13,102,104,105). A prospective randomized study was conducted by Nadig, in order to assess if the rate of unsuccessful attempts in puncturing the IJV for the placement of dialysis catheters can be reduced with the use of RTUS-guidance. In 36 punctures with RTUS-guidance only 10 unsuccessful attempts occurred, as compared to 87 unsuccessful attempts in 37 punctures using only a skin mark determined by US. Also, a reduced time to successful puncture in favour of RTUS guidance (3.4±0.9 versus 4.8±2.2 min) has been registered (106).
Conclusions
Based on this evidence, RTUS-guidance for central venous catheter placement has been endorsed as a key safety measure by both the Agency for Healthcare Quality and Research in the United States and the National Institute for Health and Care Excellence (NICE) in the UK (8,13,107-114).
Real time US guidance, examination technique
The fundamental technique of InVUS (the puncture principle) is an alignment of two planes, namely the “scan plane” that shows the target vessel on the US screen and the “needle plane” containing the needle (or other InVUS device) approaching the target. Real-time visualization of the needle tip is possible using US due to the reflection from the metal in the needle (115). The intensity of the display of echoes from the “needle plane” will depend on the needle size, the scanning depth, angulation and the US system (116). The RTUS-guidance technique can be divided into three different approaches, the longitudinal, transverse and oblique techniques.
Using the longitudinal technique, the target vessel is delineated in a long-axis view (referring to the needle: in-plane approach). With the transverse technique the target vessel is approached in a short-axis (transverse) view (referring to the needle: out-of-plane approach). Both techniques may be combined (oblique technique) (43). There is conflicting evidence with regard to the particular US-guidance technique (short-axis view/out-of-plane approach vs. long-axis view/in-plane approach), which precludes recommendation in favour of either of the two approaches (14,15,74,117-122).
Longitudinal technique
In the longitudinal technique the transducer is placed parallel to the vessel and the needle at the greatest anterio-posterior diameter of the targeted vessel. The puncture of the skin has to be close to one end of the transducer under an angle of approximately 30° from the skin surface depending from the skin-vessel distance (43). The course of the target vessel and the complete process of insertion and advancement of the needle are visualized in real-time in the long axis of the transducer (Figure 2).
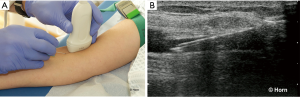
The advantage of this technique is the view of the whole needle which allows the operator to define the optimal insertion angle. By doing this the posterior wall of the vein will not be penetrated (123). However, in particular anatomical situations it may be difficult to show the course of the target vessel.
Transverse technique
In the transverse technique, also called the short-axis view, the position of the transducer is transversally placed to the vessel and the needle. The puncture of the skin should be performed exactly in the middle of the probe with an angle of approximately 45° to the skin. By tilting the probe during insertion, the tip of the needle is followed (Figure 3). The advantage of this technique is the reliable positioning of the needle tip according to the course of the vessel, preventing a deviation from the vessel’s axis to the right or left. The transverse technique is useful in anatomical areas with limited access space and for cannulation of smaller vessels. It offers more confidence for inexperienced users. In the case of unsuccessful puncture, visualisation of needle tip deviation is easy. Disadvantages are possible loss of control over the needle tip with the risk of posterior wall penetration. Posterior vessel wall penetration is a frequent event in short-axis approach to IJV cannulation (72). Moreover, it is difficult to determine the most appropriate angle for insertion.
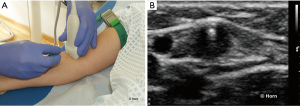
Oblique technique
In particular anatomical conditions, like puncture of the SV, the oblique technique may be helpful. It combines advantages of the short- and long-axis approaches, respectively better visualisation of the anatomical structures provided by the short-axis view and better needle tip visualization provided by the long-axis view (124).
In this approach, the position of the probe is parallel to the needle and oblique to the vessel. The view of the whole needle is maintained, while the vessel is only partially visible.
Comparison of long-axis versus short-axis vascular access
The prospective trial of Stone et al. (74) proved that the long-axis access allows improved visualization of the needle tip at the time of puncture, a result which is consistent with standard approaches of other procedures done under US-guidance (e.g., regional nerve anesthesia under US-guidance) (125,126). In this study no statistically significant differences of the time to vessel access were observed between inexperienced and experienced interventionalists (74). A recent RCT demonstrated that the long-axis access approach to the IJV and SV was more time efficient than the short-axis access. The long-axis approach to SV catheterization was also associated with fewer posterior wall penetrations (118). Disconcordantly, Blaivas et al. reported that emergency medicine residents without previous experience in US-guidance in an inanimate model were able to complete the procedure faster using the short-axis approach as compared to the long-axis approach (123).
US imaging techniques
B-mode
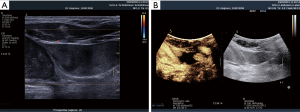
In preparation of an US-guided procedure, it is important to choose the appropriate transducer, imaging program (presetting/application) and the correct interventional apparatus (14,15). Before puncture, it is mandatory to clearly identify the vein and to rule out thrombosis, which is often done by the compressibility test using B-mode. However, in patients with very low blood pressure the artery may also be compressible. The threshold for arterial compressibility is assumed to be <60 mmHg. In patients with a very low blood flow, blood stasis may look like thrombosis using B-mode. The compressibility test may be helpful but sometimes colour Doppler imaging (CDI) and rarely CEUS are necessary to prove or rule out thrombosis (Figures 4-6). Surgical emphysema, for example in thoracic trauma, reduces the visibility of vessels. Other artifacts may be caused by circumscribed sclerosis of the arterial walls.
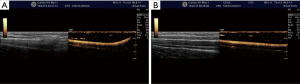
CDI
CDI may be helpful to differentiate arteries and veins and might help to identify anatomical variants and pathological findings. Compared with the landmark technique, Doppler-guidance increases the first-attempt success rate of central venous catheter placement by 58% (103). The meta-analysis of Rabindranath et al. (39) included RCTs in patients requiring hemodialysis catheter insertion. Compared to the landmark approach, RTUS Doppler-guidance significantly decreased catheter placement failure, first-attempt failure rate, time to canulation, and number of attempts per catheter insertion. Associated complications such as arterial puncture or hematoma formation were also significantly decreased. They concluded that RTUS-guidance using Doppler US should be strongly recommended for hemodialysis catheter placement.
CEUS
With the use of RTUS-guidance for catheter placement, the canulation of a thrombosed or of a small vein can be prevented (49).
CEUS is helpful:
- To diagnose thrombosis;
- To exclude thrombosis;
- For catheter tip position control;
- For detection of catheter obstruction;
- To detect pericatheter leakage.
Additional imaging
All available imaging results should be used to reduce the associated risk of vascular access. Mainly in oncological patients computed tomography and easily available US findings (21,127-129) but also the endoscopic US reports should be known (130-135).
Central venous access
General remarks
The most common used central veins for vascular access are the IJV, the femoral vein (FV) and the SV. The most appropriate central venous access site depends on the particular circumstances of the patient. As a first step, thrombosis should be ruled out. Especially for access through the IJV, it is mandatory to examine the contralateral veins since thrombosis is a contraindication to catheterisation. The IJV is the easiest central vein to puncture. On the other hand, SV access is associated with the lowest infection rate. For intravascular temperature management the FV is also a good choice. A traditional ‘blind’ approach reported failure rates of 30% or higher in emergent or cardiopulmonary arrest cases (44,136-138). In 2001, RTUS-guidance for central venous access was listed in guidelines published by the American College of Emergency Physicians as one of the primary applications for emergency US (139). Skin disinfection should be performed according to local hospital guidelines for surgical disinfection. For normal central lines, chlorhexidine is often recommended. We refer to the EFSUMB guidelines (14,15).
Anatomy
RTUS allows determination of anatomical variants, such as small diameter, medial or lateral displacement. Valsalva maneuver response, or lack thereof, needs to be correctly assessed and evaluated in order to avoid further complications.
Ultrasonographic vessel screening and imaging before vascular access
US vessel screening and imaging of the target vessels should be performed to determine the most appropriate anatomical site and the optimal patient position for central vascular access (13). In order to successfully cannulate a vessel, understanding of the technical issues is necessary. A decision upon the best approach for US-guidance (direct, indirect, free-hand, mechanical guide, Doppler) should be made by the operator, according to patient’s characteristics, equipment used and operator expertise (43). Changes in head position may influence the vein diameter and the relative position of surrounding vessels (3,140), so care must be taken (141).
Procedure
As central line insertion is painful, local anesthesia is recommended. The Seldinger procedure is normally performed. In brief, for the initial puncture a needle with attached syringe, half filled with sterile fluid, is used. After blood aspiration a guide wire is advanced under RTUS control. For the beginner, we recommend learning this procedure with another interventionalist present to aid. The second step is to perform it alone, as good coordination is required to perform the puncture with just one hand whilst manipulating the US probe with the other.
Jugular vein
Central venous access through the IJV is preferred in many cases. Due to its larger diameter it is easily accessed with wider catheters, as for hemodialysis or plasmapheresis. The rate of delayed complications, such as stenosis, is lower than for other central veins (11).
Anatomy
The IJV usually lies anterior and slightly lateral to the carotid artery, being usually larger (3), however variants are common.
Review of the literature
Denys et al. found IJV anatomical variants in 8% of the 200 patients assessed (2). Of 1,009 patients assessed by Troianos et al., in 54% of cases the IJV overlaid the carotid artery, predisposing to arterial puncture (5). Docktor et al. found the same anatomical variant in 25% of 150 patients (141). Benter et al. investigated 113 patients with haematological or oncological diseases, examining sonographically potential target regions for placement of a central catheter via the IJV and found anatomical variations of the IJV location and surrounding tissues in 36% of the patients (Figure 7). They concluded that the use of US-guided techniques for central venous catheters placement, particularly in haematological and oncological patients, is of particular importance in order to avoid arterial puncture (11). Particular attention must be payed to this group of patients because they may present a partially or completely thrombosed IJV, in up to 6% of cases according to the study of Benter et al. (11), whilst 4.4% of those investigated by Denys et al. had either thrombosed or absent IJV (2). A small IJV diameters ≤7 mm has been reported in 12–15% of the cases (142,143) and is associated with a catheterization failure rate of 14.9% (as compared to 3.9%, if IJV diameter is >7–10 mm) and a complication rate of 8.5% (as compared to 3.8%, if the IJV diameter is >7–10 mm) (143). The right IJV is as big, or bigger than the left IJV in about 74% of the patients, and offers a straighter and more direct path to the superior vena cava and the right atrium. Its cannulation is associated with a lower risk of pneumothorax, since the right lung apex is lower than the left one (142). It is worth noting that the diameter of the IJV expands during the Valsalva maneuver (144).
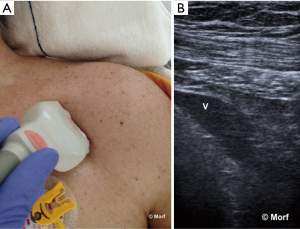
There might be differences in neonates and infants (105). Using variable degrees of head rotation, Lorchirachoonkul et al. proved that at 30° head rotation there is a potential for difficult catheterisation in 15%, with more difficulty on the left as compared to the right IJV (20% versus 10%). Head rotation did not significantly influence neither the risk of difficult catheterization, neither the size of the IJV nor the average distance between mid IJV and the skin. However, the degree of head rotation influences the position of the IJV relative to the carotid artery on both sides, with an increased overlap as the head is rotated further from the midline (142). These results have been recently confirmed by Maecken et al. only for the left IJV. These authors did not observe a significant impact of head position on the position of the right IJV (3). Therefore because anatomical variations impact on the success of IJV catheterization, as well as the incidence of associated complications, the use of RTUS-guidance is also recommended in patients with seemingly normal neck anatomy (142).
Technique and results
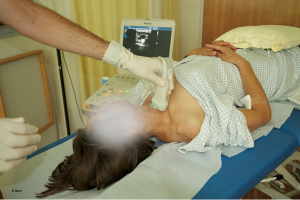
IJV catheterisation with RTUS-guidance can be performed faster, with a higher success rate (101,102) and fewer complications (33) than the traditional landmark technique (Figures 8-10).
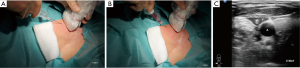
Risks and complications
Adverse events can occur even under RTUS-guidance in about 20% of the cases of IJV central line attempts (53). Complications can be classified in three categories: mechanical (with anatomical variations an important risk factor), infectious and thromboembolic (142). The most frequently encountered complication is placement of the catheter tip within the right atrium, which occurs in about 6–14% of the cases. Cardiac malposition is associated with a mortality risk due to possible cardiac perforation and subsequent tamponade (54). Pneumothorax and hemothorax are very rare if RTUS-guidance is used for central venous access (50,51). Puncture of the carotid artery is a common complication as well (Figure 11).
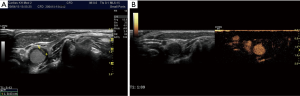
Tips and tricks (how to avoid risks and complications)
Where the IJV overlays the carotid artery, arterial puncture may occur due to the so-called “double wall puncture” phenomenon. This occurs in cases of low IJV pressure, allowing the anterior wall to be pushed against the posterior wall and the IJV to be completely compressed before the needle punctures it (100,141). A common solution is to advance the needle a little deeper and then slightly retract, until the tip lies within the IJV lumen. Exclusion of an underlying carotid artery however, is of utmost importance with this technique (100).
SV
The size of the SV allows placement of central access catheters.
Anatomy
The SV is deeply located and partially hidden under the clavicle bone. This hinders access to some of its portions. Its mid-portion can be cannulated using US guidance, however, it is difficult to obtain short-axis images at this level. Additionally, the lung apex is closely located, less than 1 cm (145), as is the subclavian artery and brachial plexus (43) with the risk of associated complications and morbidity.
Technique and results
RTUS-guidance is challenging due to the limited space available for both placement of the transducer and needle insertion. Two alternatives can be used with RTUS-guidance. One is the “low-IJV approach”, with a safer and direct route to the superior vena cava and right atrium (146). The other alternative is to access the SV further laterally on the shoulder by cannulating the axillary vein, offering a better approach under RTUS-guidance and a lower complication rate (147,148). This “axillary approach” is possible also in patients with a cervical collar or neck trauma (43). The axillary landmark approach has been proven to be safe and efficient in adults (149) and in critically ill pediatric patients (150). Using the axillary vein approach under RTUS-guidance, Gualtieri et al. obtained a higher success rate and less complications as compared to the landmark technique (92% versus 44% and 4% versus 41%, respectively), with lower mean numbers of attempts and insertion kits used (1.4 versus 2.5 and 1.0 versus 1.4, respectively) (151). In patients with relative contraindications to SV catheter placement using the landmark approach, Fry et al. (152) reported 100% success rate with RTUS-guidance.
Risks and complications
No complications were been reported by Silberzweig et al. (146) using the low-IJV approach in 116 patients. The average number of attempts needed for success was 1.2. These results have been confirmed also by the study of Milone et al., who reported no complications for the RTUS-guided cannulation of the SV, while 13% of the patients cannulated using the landmark approach developed mechanical complications (e.g., pneumothorax or arterial puncture) (153). The knowledge of surrounding structures is of main importance (154).
Detection of complications of venous access
As the EFSUMB INVUS guidelines (13) state, central venous catheter misplacement into the right heart may be detected by transabdominal US using a subxiphoidal approach or by echocardiography (55-61). Moreover, transthoracic US may be used to detect or to rule out pneumothorax related to central venous access in the critically ill patient. Therefore, routine chest radiography is dispensable after central venous line placement (54-57,62,63). Moreover, US has a very high accuracy for the detection of vascular complications of venous and arterial access, in particular of thrombosis of the target vessel (64,65), arterial pseudoaneurysm and arteriovenous fistula (66-70). Therefore, US should not only used to guide central venous access, but also to check correct placement of the line and to rule out the most common complications in the intensive care unit (13,155-157). The role of endoscopic US for catheter placement has not been examined so far (130,131,158).
Conclusions
According to the available evidence in literature it is strongly recommended to use real-time US guidance for central venous access.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Alderson PJ, Burrows FA, Stemp LI, et al. Use of ultrasound to evaluate internal jugular vein anatomy and to facilitate central venous cannulation in paediatric patients. Br J Anaesth 1993;70:145-8. [Crossref] [PubMed]
- Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med 1991;19:1516-9. [Crossref] [PubMed]
- Maecken T, Marcon C, Bomas S, et al. Relationship of the internal jugular vein to the common carotid artery: implications for ultrasound-guided vascular access. Eur J Anaesthesiol 2011;28:351-5. [Crossref] [PubMed]
- Turba UC, Uflacker R, Hannegan C, et al. Anatomic relationship of the internal jugular vein and the common carotid artery applied to percutaneous transjugular procedures. Cardiovasc Intervent Radiol 2005;28:303-6. [Crossref] [PubMed]
- Troianos CA, Kuwik RJ, Pasqual JR, et al. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology 1996;85:43-8. [Crossref] [PubMed]
- Mozersky DJ, Olson RM, Coons HG, et al. Doppler-controlled needle director: a useful adjunct to angiography. Radiology 1973;109:221-2. [Crossref] [PubMed]
- Petzoldt RK. Punktion von Venen und Arterien mittels Ultraschall. Biomedizinische Technik 1975;20:345-6.
- National Institute for Clinical Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters. London UK: NICE; 2002. Technology appraisal guidance no. 49.
- Gottschalk UD, Dietrich CF. Interventional Materials and Equipment. In: Dietrich CF, Nürnberg D. editors. Interventional Ultrasound A Practical Guide and Atlas. 1st ed. Georg Thieme Verlag: Thieme Publishers, 2014:15-33.
- Metz S, Horrow JC, Balcar I. A controlled comparison of techniques for locating the internal jugular vein using ultrasonography. Anesth Analg 1984;63:673-9. [Crossref] [PubMed]
- Benter T, Teichgraber UK, Kluhs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med 2001;22:23-6. [Crossref] [PubMed]
- Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM 2011;13:245-50. [Crossref] [PubMed]
- Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part VI - Ultrasound-Guided Vascular Interventions. Ultraschall Med 2015. [Epub ahead of print].
- Lorentzen T, Nolsoe CP, Ewertsen C, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part I. General Aspects (long Version). Ultraschall Med 2015;36:E1-14. [PubMed]
- Lorentzen T, Nolsoe CP, Ewertsen C, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part I. General Aspects (Short Version). Ultraschall Med 2015;36:464-72. [Crossref] [PubMed]
- Sidhu PS, Brabrand K, Cantisani V, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part II. Ultraschall Med 2015;36:E15-35. [Crossref] [PubMed]
- Dietrich CF, Lorentzen T, Appelbaum L, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III - Abdominal Treatment Procedures (Short Version). Ultraschall Med 2016;37:27-45. [Crossref] [PubMed]
- Fusaroli P, Jenssen C, Hocke M, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part V - EUS-Guided Therapeutic Interventions (short version). Ultraschall Med 2016;37:412-20. [Crossref] [PubMed]
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 2013;34:11-29. [PubMed]
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013;39:187-210. [Crossref] [PubMed]
- Piscaglia F, Nolsoe C, Dietrich CF, et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012;33:33-59. [Crossref] [PubMed]
- Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 2013;34:169-84. [Crossref] [PubMed]
- Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med 2013;34:238-53. [Crossref] [PubMed]
- Dietrich CF, Lorentzen T, Sidhu PS, et al. An Introduction to the EFSUMB Guidelines on Interventional Ultrasound (INVUS. Ultraschall Med 2015;36:460-3. [Crossref] [PubMed]
- Dietrich CF. EFSUMB guidelines 2015 on interventional ultrasound. Med Ultrason 2015;17:521-7. [PubMed]
- Dietrich CF. Comments and illustrations regarding the guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS)--update 2008. Ultraschall Med 2008;29 Suppl 4:S188-202. [Crossref] [PubMed]
- Dietrich CF, Chiorean L, Potthoff A, et al. Percutaneous sclerotherapy of liver and renal cysts, comments on the EFSUMB guidelines. Z Gastroenterol 2016;54:155-66. [Crossref] [PubMed]
- Dietrich CF, Cui XW, Barreiros AP, et al. EFSUMB guidelines 2011: comment on emergent indications and visions. Ultraschall Med 2012;33 Suppl 1:S39-47. [Crossref] [PubMed]
- Dietrich CF, Cui XW, Schreiber-Dietrich DG, et al. EFSUMB guidelines 2011: comments and illustrations. Ultraschall Med 2012;33 Suppl 1:S11-21. [Crossref] [PubMed]
- Randolph AG, Cook DJ, Gonzales CA, et al. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med 1996;24:2053-8. [Crossref] [PubMed]
- Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care 2006;10:R162. [Crossref] [PubMed]
- Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med 2011;39:1607-12. [Crossref] [PubMed]
- Hayashi H, Amano M. Does ultrasound imaging before puncture facilitate internal jugular vein cannulation? Prospective randomized comparison with landmark-guided puncture in ventilated patients. J Cardiothorac Vasc Anesth 2002;16:572-5. [Crossref] [PubMed]
- Cavanna L, Civardi G, Vallisa D, et al. Ultrasound-guided central venous catheterization in cancer patients improves the success rate of cannulation and reduces mechanical complications: a prospective observational study of 1,978 consecutive catheterizations. World J Surg Oncol 2010;8:91. [Crossref] [PubMed]
- Napolitano M, Malato A, Raffaele F, et al. Ultrasonography-guided central venous catheterisation in haematological patients with severe thrombocytopenia. Blood Transfus 2013;11:506-9. [PubMed]
- Serafimidis K, Sakorafas GH, Konstantoudakis G, et al. Ultrasound-guided catheterization of the internal jugular vein in oncologic patients; comparison with the classical anatomic landmark technique: a prospective study. Int J Surg 2009;7:526-8. [Crossref] [PubMed]
- Turker G, Kaya FN, Gurbet A, et al. Internal jugular vein cannulation: an ultrasound-guided technique versus a landmark-guided technique. Clinics (Sao Paulo) 2009;64:989-92. [Crossref] [PubMed]
- Rabindranath KS, Kumar E, Shail R, et al. Ultrasound use for the placement of haemodialysis catheters. Cochrane Database Syst Rev 2011.CD005279. [PubMed]
- Rabindranath KS, Kumar E, Shail R, et al. Use of real-time ultrasound guidance for the placement of hemodialysis catheters: a systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis 2011;58:964-70. [Crossref] [PubMed]
- Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessel 2014;6:13-23. [PubMed]
- Barrera R, Mina B, Huang Y, et al. Acute complications of central line placement in profoundly thrombocytopenic cancer patients. Cancer 1996;78:2025-30. [Crossref] [PubMed]
- Andel H, Rab M, Felfernig M, et al. The axillary vein central venous catheter in severely burned patients. Burns 1999;25:753-6. [Crossref] [PubMed]
- Abboud PA, Kendall JL. Ultrasound guidance for vascular access. Emerg Med Clin North Am 2004;22:749-73. [Crossref] [PubMed]
- Sznajder JI, Zveibil FR, Bitterman H, et al. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med 1986;146:259-61. [Crossref] [PubMed]
- Mansfield PF, Hohn DC, Fornage BD, et al. Complications and failures of subclavian-vein catheterization. N Engl J Med 1994;331:1735-8. [Crossref] [PubMed]
- Goldfarb G, Lebrec D. Percutaneous cannulation of the internal jugular vein in patients with coagulopathies: an experience based on 1,000 attempts. Anesthesiology 1982;56:321-3. [Crossref] [PubMed]
- Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 2001;286:700-7. [Crossref] [PubMed]
- Johnson FE. Internal jugular vein catheterization. N Y State J Med 1978;78:2168-71. [PubMed]
- Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ 2003;327:361. [Crossref] [PubMed]
- Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology 2013;118:361-75. [Crossref] [PubMed]
- Mehta N, Valesky WW, Guy A, et al. Systematic review: is real-time ultrasonic-guided central line placement by ED physicians more successful than the traditional landmark approach? Emerg Med J 2013;30:355-9. [Crossref] [PubMed]
- Leung J, Duffy M, Finckh A. Real-time ultrasonographically-guided internal jugular vein catheterization in the emergency department increases success rates and reduces complications: a randomized, prospective study. Ann Emerg Med 2006;48:540-7. [Crossref] [PubMed]
- Theodoro D, Krauss M, Kollef M, et al. Risk factors for acute adverse events during ultrasound-guided central venous cannulation in the emergency department. Acad Emerg Med 2010;17:1055-61. [Crossref] [PubMed]
- Vezzani A, Manca T, Vercelli A, et al. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound 2013;16:161-70. [Crossref] [PubMed]
- Maury E, Guglielminotti J, Alzieu M, et al. Ultrasonic examination: an alternative to chest radiography after central venous catheter insertion? Am J Respir Crit Care Med 2001;164:403-5. [Crossref] [PubMed]
- Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med 2010;38:533-8. [Crossref] [PubMed]
- Lanza C, Russo M, Fabrizzi G. Central venous cannulation: are routine chest radiographs necessary after B-mode and colour Doppler sonography check? Pediatr Radiol 2006;36:1252-6. [Crossref] [PubMed]
- Matsushima K, Frankel HL. Bedside ultrasound can safely eliminate the need for chest radiographs after central venous catheter placement: CVC sono in the surgical ICU (SICU. J Surg Res 2010;163:155-61. [Crossref] [PubMed]
- Weekes AJ, Johnson DA, Keller SM, et al. Central vascular catheter placement evaluation using saline flush and bedside echocardiography. Acad Emerg Med 2014;21:65-72. [Crossref] [PubMed]
- Bedel J, Vallee F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med 2013;39:1932-7. [Crossref] [PubMed]
- Park YH, Lee JH, Byon HJ, et al. Transthoracic echocardiographic guidance for obtaining an optimal insertion length of internal jugular venous catheters in infants. Paediatr Anaesth 2014;24:927-32. [Crossref] [PubMed]
- Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995;108:1345-8. [Crossref] [PubMed]
- Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med 2005;33:1231-8. [Crossref] [PubMed]
- Lordick F, Hentrich M, Decker T, et al. Ultrasound screening for internal jugular vein thrombosis aids the detection of central venous catheter-related infections in patients with haemato-oncological diseases: a prospective observational study. Br J Haematol 2003;120:1073-8. [Crossref] [PubMed]
- Yilmaz KB, Akinci M, Dogan L, et al. Central venous catheter-associated thrombosis in the perioperative period: a frequent complication in cancer patients that can be detected early with doppler examination. Tumori 2010;96:690-4. [PubMed]
- Ahmad F, Turner SA, Torrie P, et al. Iatrogenic femoral artery pseudoaneurysms--a review of current methods of diagnosis and treatment. Clin Radiol 2008;63:1310-6. [Crossref] [PubMed]
- Hanson JM, Atri M, Power N. Ultrasound-guided thrombin injection of iatrogenic groin pseudoaneurysm: Doppler features and technical tips. Br J Radiol 2008;81:154-63. [Crossref] [PubMed]
- Webber GW, Jang J, Gustavson S, et al. Contemporary management of postcatheterization pseudoaneurysms. Circulation 2007;115:2666-74. [Crossref] [PubMed]
- Paulson EK, Kliewer MA, Hertzberg BS, et al. Color Doppler sonography of groin complications following femoral artery catheterization. AJR Am J Roentgenol 1995;165:439-44. [Crossref] [PubMed]
- Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology 1988;168:339-42. [Crossref] [PubMed]
- Kaye AD, Fox CJ, Hymel BJ, et al. The importance of training for ultrasound guidance in central vein catheterization. Middle East J Anaesthesiol 2011;21:61-6. [PubMed]
- Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med 2009;37:2345-9. [Crossref] [PubMed]
- Moon CH, Blehar D, Shear MA, et al. Incidence of posterior vessel wall puncture during ultrasound-guided vessel cannulation in a simulated model. Acad Emerg Med 2010;17:1138-41. [Crossref] [PubMed]
- Stone MB, Moon C, Sutijono D, et al. Needle tip visualization during ultrasound-guided vascular access: short-axis vs long-axis approach. Am J Emerg Med 2010;28:343-7. [Crossref] [PubMed]
- Lutz H. Patient Information and Informed Consent. Interventional Ultrasound Conference. Berlin, 2010.
- Nuernberg DJ. Informed Consent. In: Dietrich CF. editor. Interventional Ultrasound. A Practical Guide and Atlas. New York: Georg Thieme Verlag, 2014:34-6.
- Sekhar A, Sun MR, Siewert B. A tissue phantom model for training residents in ultrasound-guided liver biopsy. Acad Radiol 2014;21:902-8. [Crossref] [PubMed]
- Barsuk JH, McGaghie WC, Cohen ER, et al. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med 2009;4:397-403. [Crossref] [PubMed]
- Barsuk JH, McGaghie WC, Cohen ER, et al. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med 2009;37:2697-701. [Crossref] [PubMed]
- Evans LV, Morse JL, Hamann CJ, et al. The development of an independent rater system to assess residents' competence in invasive procedures. Acad Med 2009;84:1135-43. [Crossref] [PubMed]
- Latif RK, Bautista AF, Memon SB, et al. Teaching aseptic technique for central venous access under ultrasound guidance: a randomized trial comparing didactic training alone to didactic plus simulation-based training. Anesth Analg 2012;114:626-33. [Crossref] [PubMed]
- Ma IW, Brindle ME, Ronksley PE, et al. Use of simulation-based education to improve outcomes of central venous catheterization: a systematic review and meta-analysis. Acad Med 2011;86:1137-47. [Crossref] [PubMed]
- Madenci AL, Solis CV, de Moya MA. Central venous access by trainees: a systematic review and meta-analysis of the use of simulation to improve success rate on patients. Simul Healthc 2014;9:7-14. [Crossref] [PubMed]
- Martiny HN. Hygiene Management. In: Dietrich CF. editor. Interventional Ultrasound: Practical Guide and Atlas. 1st ed. New York: Georg Thieme Verlag, 2014:84-7.
- Schrader G. Vaginalsonden - Einsatz und Aufbereitung. HygMed 2005;30:437-9.
- Gaillot O, Maruejouls C, Abachin E, et al. Nosocomial outbreak of Klebsiella pneumoniae producing SHV-5 extended-spectrum beta-lactamase, originating from a contaminated ultrasonography coupling gel. J Clin Microbiol 1998;36:1357-60. [PubMed]
- Hutchinson J, Runge W, Mulvey M, et al. Burkholderia cepacia infections associated with intrinsically contaminated ultrasound gel: the role of microbial degradation of parabens. Infect Control Hosp Epidemiol 2004;25:291-6. [Crossref] [PubMed]
- Jacobson M, Wray R, Kovach D, et al. Sustained endemicity of Burkholderia cepacia complex in a pediatric institution, associated with contaminated ultrasound gel. Infect Control Hosp Epidemiol 2006;27:362-6. [Crossref] [PubMed]
- Marigliano A, D'Errico MM, Pellegrini I, et al. Ultrasound echocardiographic gel contamination by Burkholderia cepacia in an Italian hospital. J Hosp Infect 2010;76:360-1. [Crossref] [PubMed]
- Muradali D, Gold WL, Phillips A, et al. Can ultrasound probes and coupling gel be a source of nosocomial infection in patients undergoing sonography? An in vivo and in vitro study. AJR Am J Roentgenol 1995;164:1521-4. [Crossref] [PubMed]
- Olshtain-Pops K, Block C, Temper V, et al. An outbreak of achromobacter xylosoxidans associated with ultrasound gel used during transrectal ultrasound guided prostate biopsy. J Urol 2011;185:144-7. [Crossref] [PubMed]
- Provenzano DA, Liebert MA, Steen B, et al. Investigation of current infection-control practices for ultrasound coupling gel: a survey, microbiological analysis, and examination of practice patterns. Reg Anesth Pain Med 2013;38:415-24. [Crossref] [PubMed]
- Schabrun S, Chipchase L, Rickard H. Are therapeutic ultrasound units a potential vector for nosocomial infection? Physiother Res Int 2006;11:61-71. [Crossref] [PubMed]
- Weist K, Wendt C, Petersen LR, et al. An outbreak of pyodermas among neonates caused by ultrasound gel contaminated with methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol 2000;21:761-4. [Crossref] [PubMed]
- Merz E. Transducer hygiene -- an underrated topic? Ultraschall Med 2005;26:7-8. [Crossref] [PubMed]
- Amis S, Ruddy M, Kibbler CC, et al. Assessment of condoms as probe covers for transvaginal sonography. J Clin Ultrasound 2000;28:295-8. [Crossref] [PubMed]
- Kac G, Podglajen I, Si-Mohamed A, et al. Evaluation of ultraviolet C for disinfection of endocavitary ultrasound transducers persistently contaminated despite probe covers. Infect Control Hosp Epidemiol 2010;31:165-70. [Crossref] [PubMed]
- German Institute for medicine and medical products (BfArM). Washing of ultrasound probes with mucosa contact. Ultraschall in Med 2005:05.
- Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV - EUS-guided Interventions: General aspects and EUS-guided sampling (Long Version). Ultraschall Med 2016;37:E33-76. [PubMed]
- Denys BG, Uretsky BF, Reddy PS. Ultrasound-assisted cannulation of the internal jugular vein. A prospective comparison to the external landmark-guided technique. Circulation 1993;87:1557-62. [Crossref] [PubMed]
- Milling TJ Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med 2005;33:1764-9. [Crossref] [PubMed]
- Ray BR, Mohan VK, Kashyap L, et al. Internal jugular vein cannulation: A comparison of three techniques. J Anaesthesiol Clin Pharmacol 2013;29:367-71. [Crossref] [PubMed]
- Brass P, Hellmich M, Kolodziej L, et al. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev 2015.CD011447. [PubMed]
- Brass P, Hellmich M, Kolodziej L, et al. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev 2015.CD006962. [PubMed]
- Hosokawa K, Shime N, Kato Y, et al. A randomized trial of ultrasound image-based skin surface marking versus real-time ultrasound-guided internal jugular vein catheterization in infants. Anesthesiology 2007;107:720-4. [Crossref] [PubMed]
- Nadig C, Leidig M, Schmiedeke T, et al. The use of ultrasound for the placement of dialysis catheters. Nephrol Dial Transplant 1998;13:978-81. [Crossref] [PubMed]
- Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001.i-x, 1-668. [PubMed]
- Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: an updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess (Full Rep) 2013.1-945. [PubMed]
- Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med 2012;38:1105-17. [Crossref] [PubMed]
- Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2011;24:1291-318. [Crossref] [PubMed]
- Jauch KW, Schregel W, Stanga Z, et al. Access technique and its problems in parenteral nutrition - Guidelines on Parenteral Nutrition, Chapter 9. Ger Med Sci 2009;7:Doc19. [PubMed]
- Pittiruti M, Hamilton H, Biffi R, et al. ESPEN Guidelines on Parenteral Nutrition: central venous catheters (access, care, diagnosis and therapy of complications. Clin Nutr 2009;28:365-77. [Crossref] [PubMed]
- Bishop L, Dougherty L, Bodenham A, et al. Guidelines on the insertion and management of central venous access devices in adults. Int J Lab Hematol 2007;29:261-78. [Crossref] [PubMed]
- AIUM practice guideline for the use of ultrasound to guide vascular access procedures. J Ultrasound Med 2013;32:191-215. [PubMed]
- Matalon TA, Silver B. US guidance of interventional procedures. Radiology 1990;174:43-7. [Crossref] [PubMed]
- Nolsøe CP, Lorentzen T, Skjoldbye BO, et al. The basics of interventional ultrasound. Ultraschall Med 2007;28:248-63; quiz 264, 267.
- Erickson CS, Liao MM, Haukoos JS, et al. Ultrasound-guided small vessel cannulation: long-axis approach is equivalent to short-axis in novice sonographers experienced with landmark-based cannulation. West J Emerg Med 2014;15:824-30. [Crossref] [PubMed]
- Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med 2015;43:832-9. [Crossref] [PubMed]
- Clemmesen L, Knudsen L, Sloth E, et al. Dynamic needle tip positioning - ultrasound guidance for peripheral vascular access. A randomized, controlled and blinded study in phantoms performed by ultrasound novices. Ultraschall Med 2012;33:E321-5. [Crossref] [PubMed]
- Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med 2011;29:1194-7. [Crossref] [PubMed]
- Sommerkamp SK, Romaniuk VM, Witting MD, et al. A comparison of longitudinal and transverse approaches to ultrasound-guided axillary vein cannulation. Am J Emerg Med 2013;31:478-81. [Crossref] [PubMed]
- Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl 2013;24:707-13. [Crossref] [PubMed]
- Blaivas M, Brannam L, Fernandez E. Short-axis versus long-axis approaches for teaching ultrasound-guided vascular access on a new inanimate model. Acad Emerg Med 2003;10:1307-11. [Crossref] [PubMed]
- Phelan M, Hagerty D. The oblique view: an alternative approach for ultrasound-guided central line placement. J Emerg Med 2009;37:403-8. [Crossref] [PubMed]
- Stone MB, Price DD, Wang R. Ultrasound-guided supraclavicular block for the treatment of upper extremity fractures, dislocations, and abscesses in the ED. Am J Emerg Med 2007;25:472-5. [Crossref] [PubMed]
- Blaivas M, Lyon M. Ultrasound-guided interscalene block for shoulder dislocation reduction in the ED. Am J Emerg Med 2006;24:293-6. [Crossref] [PubMed]
- Dietrich CF, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, part I: endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J Thorac Dis 2015;7:E311-25. [PubMed]
- Jenssen C, Annema JT, Clementsen P, et al. Ultrasound techniques in the evaluation of the mediastinum, part 2: mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography. J Thorac Dis 2015;7:E439-58. [PubMed]
- Dietrich CF, Mathis G, Cui XW, et al. Ultrasound of the pleurae and lungs. Ultrasound Med Biol 2015;41:351-65. [Crossref] [PubMed]
- Dincer HE, Gliksberg EP, Andrade RS. Endoscopic ultrasound and/or endobronchial ultrasound-guided needle biopsy of central intraparenchymal lung lesions not adjacent to airways or esophagus. Endosc Ultrasound 2015;4:40-3. [Crossref] [PubMed]
- Harris K, Modi K, Kumar A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of pulmonary artery tumors: A systematic review (with video. Endosc Ultrasound 2015;4:191-7. [Crossref] [PubMed]
- Costache MI, Iordache S, Karstensen JG, et al. Endoscopic ultrasound-guided fine needle aspiration: from the past to the future. Endosc Ultrasound 2013;2:77-85. [Crossref] [PubMed]
- Dietrich CF, Jenssen C. Endoscopic ultrasound-guided sampling in gastroenterology: European society of gastrointestinal endoscopy technical guidelines. Endosc Ultrasound 2013;2:117-22. [Crossref] [PubMed]
- Dietrich CF, Jenssen C, Arcidiacono PG, et al. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc Ultrasound 2015;4:176-90. [Crossref] [PubMed]
- Dietrich CF, Sharma M, Hocke M. Contrast-enhanced endoscopic ultrasound. Endosc Ultrasound 2012;1:130-6. [Crossref] [PubMed]
- Tripathi M. Subclavian vein cannulation: an approach with definite landmarks. Ann Thorac Surg 1996;61:238-40. [Crossref] [PubMed]
- Bo-Linn GW, Anderson DJ, Anderson KC, et al. Percutaneous central venous catheterization performed by medical house officers: a prospective study. Cathet Cardiovasc Diagn 1982;8:23-9. [Crossref] [PubMed]
- Jastremski MS, Matthias HD, Randell PA. Femoral venous catheterization during cardiopulmonary resuscitation: a critical appraisal. J Emerg Med 1984;1:387-91. [Crossref] [PubMed]
- American College of Emergency Physicians. Use of ultrasound imaging by emergency physicians. Ann Emerg Med 2001;38:469-70. [Crossref] [PubMed]
- Randall C, Schmeiser E, Fiers E, et al. Ultrasound investigation of leg position to enhance femoral vein exposure for cannulation. J Emerg Med 2014;47:176-81. [Crossref] [PubMed]
- Docktor B, So CB, Saliken JC, et al. Ultrasound monitoring in cannulation of the internal jugular vein: anatomic and technical considerations. Can Assoc Radiol J 1996;47:195-201. [PubMed]
- Lorchirachoonkul T, Ti LK, Manohara S, et al. Anatomical variations of the internal jugular vein: implications for successful cannulation and risk of carotid artery puncture. Singapore Med J 2012;53:325-8. [PubMed]
- Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer 2003;11:148-55. [PubMed]
- Weissleder R, Elizondo G, Stark DD. Sonographic diagnosis of subclavian and internal jugular vein thrombosis. J Ultrasound Med 1987;6:577-87. [PubMed]
- Skolnick ML. The role of sonography in the placement and management of jugular and subclavian central venous catheters. AJR Am J Roentgenol 1994;163:291-5. [Crossref] [PubMed]
- Silberzweig JE, Mitty HA. Central venous access: low internal jugular vein approach using imaging guidance. AJR Am J Roentgenol 1998;170:1617-20. [Crossref] [PubMed]
- Galloway S, Bodenham A. Ultrasound imaging of the axillary vein--anatomical basis for central venous access. Br J Anaesth 2003;90:589-95. [Crossref] [PubMed]
- Nickalls RW. A new percutaneous infraclavicular approach to the axillary vein. Anaesthesia 1987;42:151-4. [Crossref] [PubMed]
- Taylor BL, Yellowlees I. Central venous cannulation using the infraclavicular axillary vein. Anesthesiology 1990;72:55-8. [Crossref] [PubMed]
- Metz RI, Lucking SE, Chaten FC, et al. Percutaneous catheterization of the axillary vein in infants and children. Pediatrics 1990;85:531-3. [PubMed]
- Gualtieri E, Deppe SA, Sipperly ME, et al. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med 1995;23:692-7. [Crossref] [PubMed]
- Fry WR, Clagett GC, O'Rourke PT. Ultrasound-guided central venous access. Arch Surg 1999;134:738-40; discussion 741. [Crossref] [PubMed]
- Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann Ital Chir 2010;81:331-4. [PubMed]
- Zhan B, Zhang S, Shao Y. Operation for huge subclavian artery aneurysm: a case report. J Thorac Dis 2010;2:117-20. [PubMed]
- Refai M, Salati M, Tiberi M, et al. Clinical pathway for thoracic surgery in an Italian centre. J Thorac Dis 2016;8:S23-8. [PubMed]
- Xing X, Gao Y, Wang H, et al. Correlation of fluid balance and postoperative pulmonary complications in patients after esophagectomy for cancer. J Thorac Dis 2015;7:1986-93. [PubMed]
- Mayr NP, Michel J, Bleiziffer S, et al. Sedation or general anesthesia for transcatheter aortic valve implantation (TAVI). J Thorac Dis 2015;7:1518-26. [PubMed]
- Konge L, Colella S, Vilmann P, et al. How to learn and to perform endoscopic ultrasound and endobronchial ultrasound for lung cancer staging: A structured guide and review. Endosc Ultrasound 2015;4:4-9. [Crossref] [PubMed]