Veno-arterial extracorporeal membrane oxygenation: an overview of different cannulation techniques
Introduction
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has known a widespread application over the last decade and is now an effective and valuable therapeutic option in refractory cardiogenic shock of various etiologies (1-6). Owing to the recent European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure, short-term mechanical circulatory support (MCS) may be considered in refractory cardiogenic shock depending on patient age, comorbidities and neurological function (7). The general principle that refractory cardiogenic shock should be supported with initial temporary MCS as a “bridge to decision” before considering the patient eligible for a long-term device implantation is now well accepted worldwide. This general trend has been regularly highlighted by the annual reports of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) (8,9). The implantation of long-term MCS in cardiogenic shock patients—i.e., INTERMACS level 1 patients—fell since 2006 from 41% to 14%. As a consequence, there has been a considerable increase from 8% to 30% in the implantation of long-term MCS in INTERMACS level 3 patients—i.e., stable but inotrope-dependent heart failure patients. In this subgroup of critically ill and unstable patients in cardiogenic shock, VA-ECMO allows, on the one hand, temporary hemodynamic stabilization with improvement of end-organ function and, on the other hand, gives the time to perform complementary diagnostic exams and to decide the therapeutic strategy in these high-risk candidates for immediate long-term MCS implantation (10).
VA-ECMO could also be suggested as a rescue therapeutic option for refractory cardiac arrest. It showed promising results in the specific setting of in-hospital cardiac arrest and survival rates with good neurological outcome are reported between 20% and 40% (11-13). Conversely, there are contrasting data in the literature about survival after VA-ECMO for out-of-hospital cardiac arrest, as results are highly dependent on low-flow time (14-17).
The aim of the present paper is to offer an overview of different cannulation techniques of VA-ECMO.
Peripheral VA-ECMO
Peripheral VA-ECMO is classically accomplished through the femoral vessels. It has traditionally been performed with an open, surgical access procedure by cardiac surgeons (18). With newer vascular cannulae and insertion kits, peripheral VA-ECMO can now be implanted with a percutaneous approach, allowing for a close collaboration between cardiac surgeons, emergency department physicians, intensivists and cardiologists (19).
Surgical cannulation technique
This technique is used as the standard approach for VA-ECMO implantation by many groups and it is usually performed by cardiac surgeons.
The type of skin incision (longitudinal or transverse) is left to the surgeon’s preference. After dissection of the superficial plans, the femoral vessels are exposed enough (3–4 cm) to allow for cannulae insertion. A 4/0 polypropylene purse string is then performed on the femoral vein and artery. Before proceeding with the cannulation of the femoral vessels, it is of utmost importance to do two separate longitudinal incisions (1 cm) distally to the main surgical access. These two additional incisions represent the percutaneous exit site of the cannulae and they should be ideally performed following the longitudinal axis of the femoral vessels in order to avoid kinking or excessive traction. The subcutaneous pathway from the percutaneous exit site to the femoral vessels must be firmly dissected to allow for a harmonious introduction of the cannula without distortion of the guidewire (Figures 1,2).
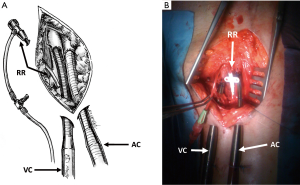
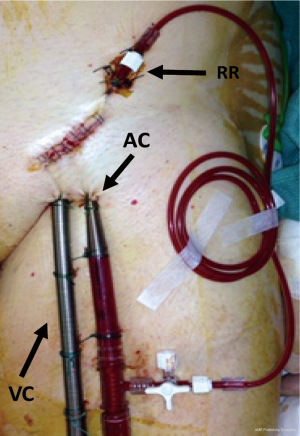
The venous (25 and 29 French) and arterial (15, 17 and 19 French) cannulae are placed using a modified Seldinger technique. This hybrid technique couples a direct surgical approach and a percutaneous technique for the insertion of guidewires and cannulae. It is strongly suggested to start with the venous cannulation. Systemic anticoagulation with 5,000 units of unfractioned heparin is usually performed at this moment before proceeding with the cannulation. During VA-ECMO implantation for refractory cardiac arrest, heparin is not administered because of the presence of coagulation abnormalities but it is started in intensive care unit after evaluation of standard coagulation laboratory exams. The puncture needle is usually introduced via the separate incision and is inserted into the femoral vein under direct vision. In obese patients the distance between the vessel and the separate exit site does not allow this maneuver; the needle is so directly introduced through the skin incision and the guidewire is then retrieved through the percutaneous exit site. The guidewire is passed through the needle and transesophageal echocardiography (TEE) confirms its correct position through the right atrium (RA) into the superior vena cava (SVC). In the cath lab, fluoroscopy can be used to visualize the wires but fluoroscopy is not mandatory in VA-ECMO cannulation (20). In some instances, as VA-ECMO implantation during cardiopulmonary resuscitation for refractory cardiac arrest, neither TEE guidance nor fluoroscopy is available. The extracorporeal membrane oxygenation (ECMO) venous cannula (VC) can be premeasured, as proposed by some authors, from the femoral insertion site to a few centimeters below the angle of Louis, approximating the junction between SVC and RA, the ideal location for the tip of a VC (21) (Figure 3). Serial dilatations of the venous puncture site are then performed and the VC is finally introduced up to the inferior vena cava-RA (IVC-RA) junction. The same principles and steps are followed for the cannulation of the femoral artery. When available, TEE checks the passage of the guidewire in the thoracic descending aorta to avoid malposition of the arterial cannula (AC). The AC must be tightly fixed to the skin before connecting the cannulae with the ECMO circuit to avoid accidental displacement or decannulation. The cannulae are then connected to the respective lines and VA-ECMO support is started. When VA-ECMO has reached steadily the theoretical flow according to the patient’s body surface area, the VC also is fixed to the skin and the purse string at the femoral vessels entry site are tied. An arterial catheter (5 or 6 French) is systematically placed distally to the entry sites of the AC to prevent lower limb ischemia. This catheter is placed in the superficial femoral artery over a 5-0 polypropylene purse string.
There are however some variants of this traditional surgical cannulation technique of femoro-femoral VA-ECMO. Other cardiac surgeons couple a percutaneous venous cannulation with a contralateral surgical arterial cannulation. Moreover, lower limb ischemia is a life-threatening complication and affects approximately 15% of VA-ECMO patients (22). An alternative technique to the direct surgical cannulation with the modified Seldinger technique is the use of a prosthetic graft that is end-to-side anastomosed to the femoral artery (23,24). The theoretical advantages of this technique are: (I) it enables blood flow through the cannula without compromising blood flow through the native artery; (II) it lowers the risk of distal leg ischemia and dissection; (III) it simplifies the decannulation procedure. The most important drawback is the potential risk of infection.
Percutaneous cannulation technique
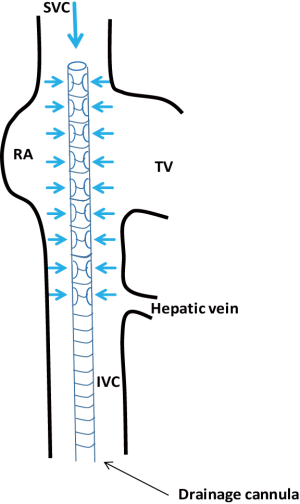
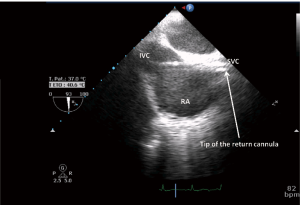
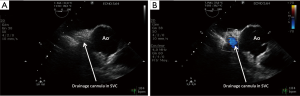
The procedure begins with the percutaneous puncture of the right femoral vein under ultrasound guidance, if available and depending on the expertise of the operator. A flexible j-tip guidewire is advanced from the femoral vein into the IVC toward the RA following a Seldinger’s technique. Attention should be paid to properly visualize the guidewire. The mid-esophageal bicaval and modified bicaval views with TEE provide excellent visualization of both venae cavae, tricuspid valve and RA. The guidewire should be ideally seen in both cavae to confirm that it has not passed through the tricuspid valve into the right ventricle, across an atrial septal defect or into the coronary sinus. Serial dilatations of the venous puncture site are then performed and the VC is finally introduced up to the SVC-RA junction. During the repeated dilatation of the skin and subcutaneous tissues, and whilst threading the cannula over the guidewires, it is necessary to maintain visualization of the guidewire to identify any secondary migration (25). Attention should also be paid to monitoring any new or increasing pericardial collection (26). TEE confirms the correct position of the drainage cannula (Figures 4,5).
For percutaneous VA-ECMO implantation we recommend to puncture the contralateral femoral artery (Figure 6) (27). The same principles and steps are followed for the cannulation of the femoral artery. When available, TEE checks the passage of the guidewire in the thoracic descending aorta.
A 5 or 6 French percutaneous catheter should be placed distal to the AC entry site into the superficial femoral artery to prevent lower limb ischemia (Figure 6). The superficial femoral artery is identified under ultrasound guidance. The guidewire is then inserted and directed toward the foot for distal perfusion catheter placement. Close surveillance of limb perfusion is crucial to avoid disastrous ischemic complications. Clinical evaluation, and echo-Doppler ultrasonography of limb vessels are mandatory. Recently, it has been demonstrated that near-infrared spectroscopy is another valuable method to monitor tissue oxygenation of lower extremities in VA-ECMO patients (28).
Open repair when decannulating is recommended in each VA-ECMO peripheral cannulation technique, as unexpected bleeding, hematoma, pseudoaneurysm or arteriovenous fistula may occur. The venous cannulation site is closed with purse string stitches. Doppler examination and occasionally Fogarty thrombectomy are necessary. In a recent study, a percutaneous technique using two ProGlide® devices (Abbott Vascular, Redwood City, CA, USA) by direct puncture of an AC at the time of weaning off ECMO showed that this technique was a feasible and safe strategy for weaning from percutaneous VA-ECMO in particular patients with smaller cannula ranged from 14 to 18 F. This method was not inferior to standard open femoral exposure. However, large-scale, prospective, randomized, controlled trials are needed to clarify the efficacy and safety of the double ProGlide-assisted closure strategy in patients with VA-ECMO support (29).
Alternative peripheral cannulation techniques
The cannulation of the femoral artery for the implantation of VA-ECMO displays significant risks such as distal limb ischemia, dissection, thrombosis, embolization, retroperitoneal hemorrhage and potential brain and myocardial ischemia as a result of retrograde perfusion. Moreover, in some instances like ilio-femoral severe peripheral vascular disease or previous vascular surgery, the femoral access route is contraindicated.
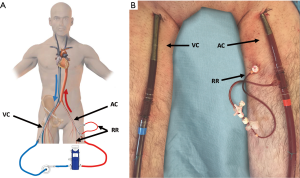
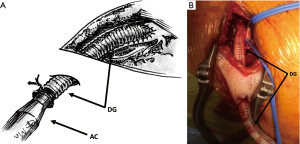
The subclavian artery as an alternative approach to peripheral VA-ECMO has been first described in 2003 (30). The subclavian artery is usually exposed at the deltoid-pectoral groove, preferentially at the right side. The skin incision is performed below and parallel to the lateral two thirds of the clavicle (Figure 7). Some authors prefer a more medial approach, claiming a lower risk of brachial plexus injury (31). The pectoralis major muscle is divided and the pectoralis minor is retracted or sectioned. Further dissection allows for the identification of the subclavian artery and its distinction from the brachial plexus. The exposure of the artery is improved by passing proximal and distal shoestring ties around it. After heparin administration and arterial clamping, a 1 cm arteriotomy is created and an 8 mm Dacron graft (DG) is sewn in an end-to-side fashion. As previously described a 45° oblique anastomosis rather than in a perpendicular fashion is strongly suggested to have a more laminar flow across the subclavian artery and reduce the risk of upper extremity edema (32). In some cases the anastomosis and the graft may be reinforced with biological glue. In order to reduce accidental damages and infectious complications, the vascular graft is subcutaneously tunneled and exteriorized 4 to 5 cm downward with a separate surgical incision. The AC (usually 18 or 20 French) is introduced in the graft and advanced as far as possible to within 1 cm of the anastomosis. The cannula is finally secured to the graft and fixed to the patient’s chest. When the device will be decannulated, the AC is removed, the DG is cut leaving a 1 cm cuff and the remaining cuff is over-sewn.
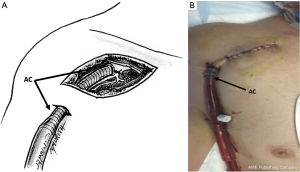
Otherwise, direct cannulation can also be performed (33,34) (Figures 7,8). A single 5-0 polypropylene purse-string suture is placed on the anterior wall of the artery. A separate skin incision (1 cm) is done downward to the main surgical access. This additional incision represents the percutaneous exit site of the cannula and it should be ideally performed following the longitudinal axis of the vessel. Cannulation is then performed using the modified Seldinger technique with progressive dilatation of the artery. A further perfusion catheter could be inserted in the distal subclavian artery and connected to the arterial line in order to reduce the risk of arm ischemia, especially in those patients with expected longer period of support (35).
In the subclavian VA-ECMO femoral venous cannulation is performed percutaneously. Alternatively the right internal jugular vein could be cannulated percutaneously as well, allowing for early mobilization and physical rehabilitation of the patients (36).
The subclavian VA-ECMO configuration displays some advantages and drawbacks: (I) the subclavian artery is rarely affected by atherosclerotic lesions compared to femoral artery; (II) the presence of a rich collateral flow reduces the risk of upper extremity ischemia; (III) bacterial contamination is less likely in this anatomic region; (IV) it provides a systemic antegrade perfusion; (V) the surgical dissection could be technical challenging in obese patients or in case of excessive chest wall edema; (VI) it cannot be used in highly unstable cardiogenic shock patients or for refractory cardiac arrest during cardiopulmonary resuscitation as it is time-consuming.
To the best of our knowledge, the “Cleveland Clinic” group published to date the largest series of subclavian VA-ECMO (n=81) using the side graft cannulation technique (37). In this retrospective analysis, hyperperfusion syndrome with edematous limb was the most common complication involving 25% of the patient population. Moreover, bleeding from the cannulation site requiring surgical re-exploration was statistically more frequent after subclavian artery cannulation (17%) rather than femoral or central cannulation (5% and 1%, respectively). These drawbacks were counterbalanced by the low rate of upper limb ischemia (1%) as compared to the rate of lower limb ischemia of the femoral cannulation (16%).
Central VA-ECMO
Central, intrathoracic VA-ECMO is less frequently used as compared to the peripheral configuration. It is usually reserved to postcardiotomy cardiogenic shock or primary graft failure after heart transplantation as standard sternotomy has already been performed. Moreover, central VA-ECMO is an effective option to unload the left ventricle (LV) in peripheral femoro-femoral VA-ECMO patients developing pulmonary edema. Although some centers use an intraaortic balloon pump in conjunction with peripheral VA-ECMO to reduce LV and pulmonary congestion, no definitive data exist to support its routine use.
LV and aortic root stasis from lack of cardiac ejection and failure of aortic valve opening may result in catastrophic intracardiac and aortic root thrombosis. Increased anticoagulation to minimize this risk may heighten the risk of significant bleeding.
Minimally invasive strategies such as percutaneous transseptal left atrial decompression (38) and subxiphoid surgical approaches to drain the LV (39) have been described to reduce LV distension. The residual atrial defect may require correction once the patient has been weaned from mechanical support. Use of a percutaneously inserted VAD (Impella™, Abiomed, Aachen, Germany) to decompress the LV has also been reported in this setting (40), alleviating the need for a high-risk septostomy or surgical venting. Femoral VA-ECMO is also limited by femoral arterial size, and thus cannula size and the requirement for distal limb perfusion. Given its less invasive nature (compared with thoracic access), peripheral VA-ECMO—with attention to optimal LV afterload, minimising LV distension with optimal fluid and inotrope therapy, anticoagulation and pulmonary management—is a viable first-line option for patients with isolated acute cardiac failure refractory to conventional management. The limitations of peripheral VA-ECMO have prompted the use of ECMO devices (41) to facilitate ventricular unloading by changing to a temporary left ventricular assist device (LVAD) or a biventricular assist device configuration. Any perfusion strategy that creates a right to left shunt requires an oxygenator in the circuit. Oxygenators may additionally provide temperature control. This strategy effectively provides biventricular support and gas exchange through a single pump configuration with the ability to cease right ventricular (RV) support when not required. However, this configuration requires sternotomy and cannulation of the LV (or left atrium) and aorta. A reoperation (sternotomy or thoracotomy) is then required for explantation of the cannulae upon cardiac recovery or for implantation of a long-term mechanical assist device (42). Less invasive techniques for temporary cardiorespiratory support including a transition strategy to an intermediate-term support configuration allowing mobilization have been described (43). Although this configuration requires a left thoracotomy, sternotomy is avoided, potentially reducing risk for subsequent surgery in the absence of cardiac recovery (long-term VAD implantation as a bridge to destination or heart transplantation).
VA-ECMO cannot provide specific LV unloading without modification. Where cardiogenic shock is associated with isolated acute LV failure, absent LV ejection with any aortic valve regurgitation or severe aortic agree or mitral valve regurgitation (i.e., any situation in which regurgitant volume exceeds stroke volume), VA-ECMO can exacerbate pulmonary edema and induce fatal pulmonary hemorrhage (44). In these circumstances, even if VA-ECMO can be reconfigured to include surgical or percutaneous drainage of the LV, or substituted with an alternative form of cardiac support (45), central cannulation with LV decompression remains the gold standard treatment (46).
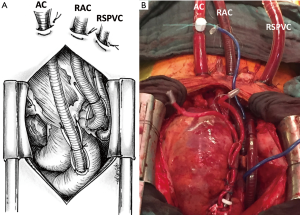
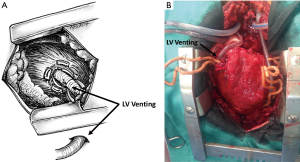
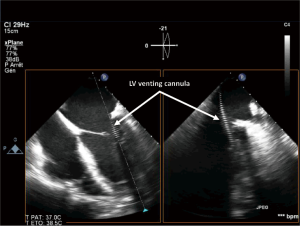
The surgical technique we describe as follows represents our institutional policy for the implantation of central VA-ECMO (Geneva University Hospital and “Louis Pradel” Cardiologic Hospital, Lyon). A 18–20 French AC is inserted using the modified Seldinger technique into the ascending aorta. The site of cannulation should be quite proximal allowing for long-term MCS implantation or heart transplantation in the absence of myocardial recovery. The RA has been classically cannulated for venous drainage but, in our opinion, the cannulation of the RA is no more necessary owing to the large diameter (25 or 29 French) of available femoral VCs. Finally, a heparin-coated 16–20 French venting cannula is inserted through the LV apex or the right superior pulmonary vein into the LV (Figures 9,10). Some authors suggested a larger diameter cannula to unload the LV (46). The correct position of the tip of the unloading cannula in the LV is checked under TEE guidance and it is connected to the ECMO venous line using a Y-connector (Figure 11).
Conclusions
VA-ECMO is an effective therapeutic option in cardiogenic shock patients refractory to optimal maximal medical treatment. It could also be suggested as a rescue therapeutic option for refractory cardiac arrest. Peripheral femoro-femoral VA-ECMO is the first-line technique in consideration of its ease of implantation and decannulation. The sublclavian artery is an alternative appealing approach in those patients in whom the surgical access of ilio-femoral vessels is contraindicated. Central intrathoracic VA-ECMO is usually reserved to postcardiotomy cardiogenic shock or primary graft failure after heart transplantation as standard sternotomy has already been performed. Finally, central VA-ECMO is an effective option to unload the LV in peripheral femoro-femoral VA-ECMO patients developing refractory pulmonary edema.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Arroyo D, Gasche Y, Banfi C, et al. Successful heart transplantation after prolonged cardiac arrest and extracorporeal life support in organ donor-a case report. J Cardiothorac Surg 2015;10:186. [Crossref] [PubMed]
- Brunner ME, Siegenthaler N, Shah D, et al. Extracorporeal membrane oxygenation support as bridge to recovery in a patient with electrical storm related cardiogenic shock. Am J Emerg Med 2013;31:467.e1-6. [Crossref] [PubMed]
- Giraud R, Banfi C, Siegenthaler N, et al. Massive pulmonary embolism leading to cardiac arrest: one pathology, two different ECMO modes to assist patients. J Clin Monit Comput 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Giraud R, Siegenthaler N, Tassaux D, et al. When the heart and/or the lung fails: the ECMO. Rev Med Suisse 2011;7:2444-51. [PubMed]
- Pavlovic G, Banfi C, Tassaux D, et al. Peri-operative massive pulmonary embolism management: is veno-arterial ECMO a therapeutic option? Acta Anaesthesiol Scand 2014;58:1280-6. [Crossref] [PubMed]
- Pozzi M, Banfi C, Grinberg D, et al. Veno-arterial extracorporeal membrane oxygenation for cardiogenic shock due to myocarditis in adult patients. J Thorac Dis 2016;8:E495-502. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant 2012;31:117-26. [Crossref] [PubMed]
- Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495-504. [Crossref] [PubMed]
- Lebreton G, Pozzi M, Mastroianni C, et al. Extracorporeal life support as a bridge to bridge: a strategy to optimize ventricular assist device results. Eur J Cardiothorac Surg 2015;48:785-91. [Crossref] [PubMed]
- Chen YS, Chao A, Yu HY, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol 2003;41:197-203. [Crossref] [PubMed]
- Chen YS, Lin JW, Yu HY, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 2008;372:554-61. [Crossref] [PubMed]
- Wang CH, Chou NK, Becker LB, et al. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest--a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation 2014;85:1219-24. [Crossref] [PubMed]
- Maekawa K, Tanno K, Hase M, et al. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med 2013;41:1186-96. [Crossref] [PubMed]
- Pozzi M, Koffel C, Armoiry X, et al. Extracorporeal life support for refractory out-of-hospital cardiac arrest: Should we still fight for? A single-centre, 5-year experience. Int J Cardiol 2016;204:70-6. [Crossref] [PubMed]
- Le Guen M, Nicolas-Robin A, Carreira S, et al. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Crit Care 2011;15:R29. [Crossref] [PubMed]
- Rousse N, Robin E, Juthier F, et al. Extracorporeal Life Support in Out-of-Hospital Refractory Cardiac Arrest. Artif Organs 2016;40:904-9. [PubMed]
- Schmid C, Philipp A, Mueller T, et al. Extracorporeal life support - systems, indications, and limitations. Thorac Cardiovasc Surg 2009;57:449-54. [Crossref] [PubMed]
- Conrad SA, Grier LR, Scott LK, et al. Percutaneous cannulation for extracorporeal membrane oxygenation by intensivists: a retrospective single-institution case series. Crit Care Med 2015;43:1010-5. [Crossref] [PubMed]
- Douflé G, Roscoe A, Billia F, et al. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care 2015;19:326. [Crossref] [PubMed]
- Lamb KM, Hirose H, Cavarocchi NC. Preparation and technical considerations for percutaneous cannulation for veno-arterial extracorporeal membrane oxygenation. J Card Surg 2013;28:190-2. [Crossref] [PubMed]
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6. [Crossref] [PubMed]
- Vander Salm TJ. Prevention of lower extremity ischemia during cardiopulmonary bypass via femoral cannulation. Ann Thorac Surg 1997;63:251-2. [Crossref] [PubMed]
- Calderon D, El-Banayosy A, Koerner MM, et al. Modified T-Graft for Extracorporeal Membrane Oxygenation in a Patient with Small-Caliber Femoral Arteries. Tex Heart Inst J 2015;42:537-9. [Crossref] [PubMed]
- Yastrebov K, Manganas C, Kapalli T, et al. Right ventricular loop indicating malposition of J-wire introducer for double lumen bicaval venovenous extracorporeal membrane oxygenation (VV ECMO) cannula. Heart Lung Circ 2014;23:e4-7. [Crossref] [PubMed]
- Hirose H, Yamane K, Marhefka G, et al. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg 2012;7:36. [Crossref] [PubMed]
- Banfi C, Bendjelid K, Giraud R. Conversion from percutaneous venoarterial extracorporeal membrane oxygenation access to a peripheral arterial cannulation: is it safe? J Thorac Cardiovasc Surg 2014;147:1995-6. [Crossref] [PubMed]
- Steffen RJ, Sale S, Anandamurthy B, et al. Using near-infrared spectroscopy to monitor lower extremities in patients on venoarterial extracorporeal membrane oxygenation. Ann Thorac Surg 2014;98:1853-4. [Crossref] [PubMed]
- Hwang JW, Yang JH, Sung K, et al. Percutaneous removal using Perclose ProGlide closure devices versus surgical removal for weaning after percutaneous cannulation for venoarterial extracorporeal membrane oxygenation. J Vasc Surg 2016;63:998-1003.e1. [Crossref] [PubMed]
- Moazami N, Moon MR, Lawton JS, et al. Axillary artery cannulation for extracorporeal membrane oxygenator support in adults: an approach to minimize complications. J Thorac Cardiovasc Surg 2003;126:2097-8. [Crossref] [PubMed]
- Baribeau YR, Westbrook BM, Charlesworth DC. Axillary cannulation: first choice for extra-aortic cannulation and brain protection. J Thorac Cardiovasc Surg 1999;118:1153-4. [Crossref] [PubMed]
- Pocar M, Moneta A, Mattioli R, et al. Closed-chest transaxillary venoarterial ECMO. Ann Thorac Surg 2006;81:1177-8; author reply 1178. [Crossref] [PubMed]
- Navia JL, Atik FA, Beyer EA, et al. Extracorporeal membrane oxygenation with right axillary artery perfusion. Ann Thorac Surg 2005;79:2163-5. [Crossref] [PubMed]
- Hysi I, Fabre O, Renaut C, et al. Extracorporeal membrane oxygenation with direct axillary artery perfusion. J Card Surg 2014;29:268-9. [Crossref] [PubMed]
- Capuano F, Danesi TH, Roscitano A, et al. How to ensure a good flow to the arm during direct axillary artery cannulation. Eur J Cardiothorac Surg 2011;40:520-1. [PubMed]
- Biscotti M, Bacchetta M. The "sport model": extracorporeal membrane oxygenation using the subclavian artery. Ann Thorac Surg 2014;98:1487-9. [Crossref] [PubMed]
- Chamogeorgakis T, Lima B, Shafii AE, et al. Outcomes of axillary artery side graft cannulation for extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg 2013;145:1088-92. [Crossref] [PubMed]
- Aiyagari RM, Rocchini AP, Remenapp RT, et al. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med 2006;34:2603-6. [Crossref] [PubMed]
- Guirgis M, Kumar K, Menkis AH, et al. Minimally invasive left-heart decompression during venoarterial extracorporeal membrane oxygenation: an alternative to a percutaneous approach. Interact Cardiovasc Thorac Surg 2010;10:672-4. [Crossref] [PubMed]
- Vlasselaers D, Desmet M, Desmet L, et al. Ventricular unloading with a miniature axial flow pump in combination with extracorporeal membrane oxygenation. Intensive Care Med 2006;32:329-33. [Crossref] [PubMed]
- Aggarwal A, Modi S, Kumar S, et al. Use of a single-circuit CentriMag® for biventricular support in postpartum cardiomyopathy. Perfusion 2013;28:156-9. [Crossref] [PubMed]
- Shekar K, Mullany DV, Thomson B, et al. Extracorporeal life support devices and strategies for management of acute cardiorespiratory failure in adult patients: a comprehensive review. Crit Care 2014;18:219. [Crossref] [PubMed]
- Massetti M, Gaudino M, Saplacan V, et al. From extracorporeal membrane oxygenation to ventricular assist device oxygenation without sternotomy. J Heart Lung Transplant 2013;32:138-9. [Crossref] [PubMed]
- Pellegrino V, Hockings LE, Davies A. Veno-arterial extracorporeal membrane oxygenation for adult cardiovascular failure. Curr Opin Crit Care 2014;20:484-92. [Crossref] [PubMed]
- Soleimani B, Pae WE. Management of left ventricular distension during peripheral extracorporeal membrane oxygenation for cardiogenic shock. Perfusion 2012;27:326-31. [Crossref] [PubMed]
- Weymann A, Schmack B, Sabashnikov A, et al. Central extracorporeal life support with left ventricular decompression for the treatment of refractory cardiogenic shock and lung failure. J Cardiothorac Surg 2014;9:60. [Crossref] [PubMed]


