Extensive plastic bronchitis: etiology of a rare condition
Introduction
Bronchial casts are a rare complication seen in diseases with increased volume or viscosity of bronchial secretions. Bronchial casts have also been documented in patients with cardiac valvular abnormalities and amyloidosis (1). This type of complication is more often seen in children than adults.
We present the case of a 55-year-old patient with esophageal cancer and who underwent trachea-esophageal (TE) fistula repair. Throughout his hospital stay, the patient exhibited difficulty breathing and showed increasing opacities on chest X-rays. On post-operative days 32–35, occlusive bronchial casts were extracted and analyzed. It is still unknown why this rare complication occurred, but we discuss the factors that likely played a role.
Case presentation
A 55-year-old man presented with a T3N2M0 distal esophageal adenocarcinoma. The patient underwent neoadjuvant chemoradiation, followed by a robotic-assisted Ivor Lewis esophagectomy 4 months after diagnosis. A post-operative contrast esophagogram demonstrated no anastomotic leak, and he started drinking liquids. Three weeks after this procedure, the patient returned to the Thoracic Surgery clinic complaining of cough and vomiting. Initially, he was believed to have delayed gastric emptying and underwent an esophagogastroduodenoscopy (EGD) with dilation and Onabotulinum Toxin A (Botox) (Allergan, Dublin, Ireland) injection of the pylorus. A 2-mm opening at the esophagogastric anastomosis and a small TE fistula were also discovered. At that time, it was decided to observe the small fistula.
After a few days, the patient presented with increasing dyspnea, productive cough, and leukocytosis. It was decided to attempt to seal the TE fistula with an esophageal stent—a less invasive approach than a surgical repair. A covered 70-mm × 18-mm Alimaxx® esophageal stent (Merit Medical Endotek, South Jordan, UT, USA) was placed over the TE fistula, but the stent migrated distally into the gastric conduit. Therefore, a larger-diameter covered 70-mm × 22-mm Alimaxx esophageal stent was placed in a second attempt to close the TE fistula, and an endoscopic clip was placed to hold the stent in position. However, a week later, his symptoms worsened, and a nasogastric (NG) tube was placed to decompress the gastric conduit. Therapeutic bronchoscopy with suctioning and EGD were performed.
During the flexible EGD, the esophageal stent was observed to be patent and in position, and the NG tube was going through the esophageal stent into the distal gastric conduit. No gastric conduit outlet obstruction was seen.
At bronchoscopy, copious amounts of thin, clear mucus-like bronchial secretions were found and suctioned. The TE fistula had significantly worsened. A 20-mm ischemic defect was identified through the membranous portion of the proximal right mainstem bronchus, and a 2-mm hole through the membranous portion of the left mainstem bronchus was also found. These two defects were at the level of the esophagogastric anastomosis.
After discussing the findings with the patient and his family, surgical repair of the TE fistula was recommended. The patient underwent a bronchoscopy, EGD, and right thoracotomy.
During the bronchoscopy, significant amounts of clear mucus emanating into the airway from the TE fistula was suctioned. During the thoracotomy, defects were found in the right and left mainstem bronchi at the level of the carina, but no empyema or pleural effusions are observed. The metal endoscopic clip was noted to have eroded through the esophagus and into membranous portion of the right mainstem bronchus. Consequently, the endoscopic clip and esophageal stent were removed.
The esophagogastric anastomosis was repaired with five simple-interrupted 3-0 silk stitches. An NG tube was passed. Simple 3-0 Vicryl stitches were also placed to close the membranous defect of the left mainstem bronchus. The 2-cm right mainstem bronchial defect was repaired with biological mesh (Strattice®, Lifecell Corporation, Branchburg, NJ, USA). The mesh was sutured to the membranous portion of the distal trachea and extending onto both the right and left mainstem bronchi. A pedicled latissimus dorsi muscle flap was harvested and placed between the mesh and the esophagogastric anastomosis. No air leak was observed after the tracheobronchial defect repair.
On post-operative day (POD) 3, the patient vomited 1 L of bile and aspirated. On POD 4, he developed tachypnea, hypoxia and coarse rhonchi bilaterally on auscultation.
On POD 5, the patient’s respiratory distress worsened, and intubation and mechanical ventilation were required. Shortly after intubation, bronchoscopy was performed and revealed small mucus plugs and an intact tracheobronchial surgical repair. Chest X-ray showed bilateral atelectasis and mild perihilar infiltrates. That night, his hypoxia suddenly worsened. Repeat chest X-ray showed worsening patchy opacities in both lungs. A second bronchoscopy was performed and showed once again an intact surgical repair with the biological mesh in place. The right-sided airway was patent, but the distal left mainstem bronchus was 90% occluded from small mucous plugs, which were suctioned out. A cervical tracheostomy was performed to help with management of airway secretions in the intensive care unit.
Within the next 24 hours, it became more difficult to ventilate the patient due to high peak pressures. An emergency flexible and rigid bronchoscopy was performed. This time, rather than small mucous plugs, very long, rubbery bronchial casts were found. These occlusive casts were filling the entire airway from the distal trachea to the sub-segmental bronchi bilaterally. They could only be retrieved through a rigid scope using large forceps.
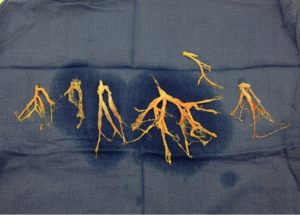
Examination of the bronchial casts in pathology showed oblong fragments of tan, rubbery tissue measuring 4 cm × 2 cm × 0.6 cm in aggregate (Figures 1 and 2). Histologically, the casts consisted of admixed mucus, blood, fibrin, neutrophils, and benign squamous cells, with associated bacteria. The bacteria resembled Actinomyces species. No atypia or malignancy was seen. Cultures of the casts and sputum grew 2+ Enterococcus species and Candida glabrata.
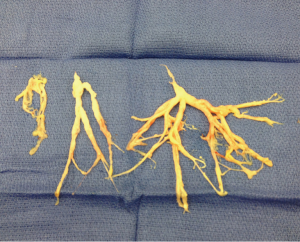
Over the next 48 hours, the patient formed these occlusive bronchial casts rapidly, leading to an inability to ventilate him. He required emergent evacuation of these occlusive bronchial casts with a rigid bronchoscope 5 times within a 48-hour period.
Within this time period, the patient developed a rapidly progressive systemic inflammatory response, despite high dose steroids, antibiotics, and anti-fungal medications. He developed anuric renal failure requiring dialysis as well as disseminated intravascular coagulation (DIC) with anemia and thrombocytopenia. After discussion with the family, he was terminally extubated and consequently expired.
Discussion
Plastic bronchitis, or bronchitis plastica, is a rare condition where bronchial casts are formed. These bronchial casts are removed either by expectoration or bronchoscopy. Bronchitis plastica is more common in children than in adults and usually occurs secondary to an underlying pulmonary or cardiovascular disease. Bronchial casts have been associated with diseases that increase volume or viscosity of bronchial secretions, such as bronchopulmonary bacterial infection, tuberculosis, asthma, cystic fibrosis, inhalation of foreign bodies, allergic bronchopulmonary Aspergillosis, bronchiectasis, and chronic bronchitis. Bronchial cast formation has been documented in cases of lymphatic leakage into bronchi (2). Bronchial casts have also been documented in patients with cardiac valvular abnormalities, constrictive pericarditis, cyanotic congestive heart disease, sickle-cell anemia, vascular stasis, and amyloidosis (2,3). Bronchitis plastica is a known complication to the Fontan procedure for congenital cardiac anomaly, and bronchial casts are believed to be formed by the inflammation caused by heart surgery, which triggers an increase in volume of bronchial secretions and venous pressure (2,4).
The bronchial casts are classified into two types based on histology. Type I is also known as inflammatory or cellular type, because the casts have eosinophils, fibrin, and Charcot-leiden crystals. This type usually presents acutely. In contrast, Type II, acellular or non-inflammatory type, has mucin and exhibits vascular hydrostatic changes. This latter type is usually seen with underlying cardiac diseases. Type-II pathogenesis could be from extravasation of serum proteins into airways in the presence of an abnormal vascular tree or disordered pulmonary lymphatic drainage. Type-II casts are seen chronically (1-3,5-7).
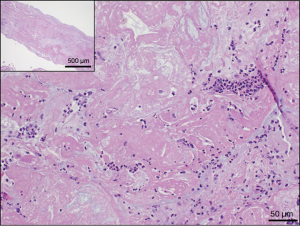
In our patient’s case, the microscopic examination of all the bronchial casts removed showed tubular/cylindrical structures composed primarily of fibrin and mucous, with embedded inflammatory cells (Figure 3). In histology, hematoxylin and eosin staining of the pathology slides gives a shaded pink appearance to the fibrin, and mucous appears pale gray. Inflammatory cells consisting of neutrophils, lymphocytes, macrophages and rare eosinophils are embedded within the casts and are easily visible due to the deep purple nuclear staining of these cells. Red blood cells appear bright red when present, and rare benign squamous cells are sometimes present embedded in the fibrin and mucous as well. The type of cast seen in our patient can be classified as Type-I. The classification of plastic bronchitis has been proposed to be changed to allergic/asthmatic, cardiac, and idiopathic in order to correlate with associated disease states (4).
The Type-I, or inflammatory, casts are formed when there is an inflammatory response, either due to an infectious agent, allergic reaction, or trauma. For our patient, we believe that this rare complication was due to multiple factors: aspiration pneumonia, erosion of the esophageal stent and metallic clip, inflammation of the biological mesh used to repair the TE fistula as well as trauma from mechanical ventilation.
Aspiration was suspected throughout the patient’s peri-operative period. He also had a witnessed massive aspiration of bile during his hospital stay which was treated with antibiotics, antifungals, and steroids. In addition to this, bile and gastric secretions were contaminating the airways through the TE fistula causing a chemical inflammation.
Another factor that contributed to an exaggerated inflammatory response was his Candida glabrata and Enterococcus faecium bronchial infection. There have been several cases in which pulmonary infections, such as Influenza H1N1 virus, have triggered the creation of bronchial casts. However, a pulmonary yeast infection has not been reported as a triggering factor. In other documented cases, underlying chronic conditions, such as asthma, are suggested to place patients at higher risk. Our patient did not report any chronic disease, except esophageal cancer.
Infectious organisms are not the only factors that cause bronchial cast formation (6). Another factor that could have played a role in bronchial cast formation was the inflammation and chemical interaction between the bile, gastric secretion, and infected mucus with the biological mesh used for the surgical repair of the TE fistula. The Strattice mesh consists of a sterilized sheet of processed porcine dermis. This de-cellularized porcine dermis is designed to incorporate into the recipient tissue and serves as a matrix for cellular and microvascular ingrowth. Although designed to be inert, it is not known if exposure of this mesh to the infectious and chemical elements in this patient’s airway contributed to the aggressive production of bronchial casts.
One last factor that we believe played a role in bronchial cast formation was the extended period of intubation. The tracheal trauma by continued mechanical ventilation could cause tracheobronchitis due to barotrauma and delivery of high concentrations of oxygen, which leads to loss of ciliary epithelium and impaired mucus flow (3). Our patient required intubation for a significant number of days during his hospital stay, which could have played a role in the formation of bronchial casts. Although prolonged mechanical ventilation is common in the intensive care patient population, extensive occlusive bronchial cast formation is exceedingly rare.
Conclusions
In our patient’s case, the bronchial cast formation was due to an inflammatory response. We believe that the Candida glabrata and Enterococcus faecium bronchial infection, repeated bile and gastric content aspiration, interaction of the biological mesh with local airway factors and the extended intubation were all factors, leading to the aggressive formation of occlusive bronchial casts and leading to his demise.
Acknowledgements
This research was supported in part by funding to MF Echavarria from the Scholarly Concentrations Program at University of South Florida (USF) Health, Morsani College of Medicine.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Migliore M, Ciancio N, Giuliano R, et al. Bronchial cast hiding a lung cancer. Multidiscip Respir Med 2012;7:43. [Crossref] [PubMed]
- Somani SS, Naik CS. Bronchial cast: a case report. Indian J Otolaryngol Head Neck Surg 2008;60:242-4. [Crossref] [PubMed]
- Ferreres-Franco J, Blanquer-Olivas J, Pastor-Esplá E, et al. Intermittent asphyxia syndrome caused by a bronchial cast in the subglottic region. Arch Bronconeumol 2005;41:638-40. [PubMed]
- Madsen P, Shah SA, Rubin BK. Plastic bronchitis: new insights and a classification scheme. Paediatr Respir Rev 2005;6:292-300. [Crossref] [PubMed]
- Vijayasekaran D, Sambandam AP, Gowrishankar NC. Acute plastic bronchitis. Indian Pediatr 2004;41:1257-9. [PubMed]
- Hasegawa M, Inamo Y, Fuchigami T, et al. Bronchial casts and pandemic (H1N1) 2009 virus infection. Emerg Infect Dis 2010;16:344-6. [Crossref] [PubMed]
- Kovesi T, Gardin L. Bronchial cast. Pediatr Cardiol 2012;33:675-6. [Crossref] [PubMed]