Emerging co-morbidities of obstructive sleep apnea: cognition, kidney disease, and cancer
Introduction
Obstructive sleep apnea (OSA) is a chronic, highly prevalent sleep disorder, with recent prevalence estimates of around 22% in men and 17% in women (1). Despite progress in raising public awareness of OSA, it remains underdiagnosed in the general population (2,3). Episodes of intermittent hypoxia (IH) and sleep fragmentation occur during nocturnal cycles of pharyngeal collapse, leading to a cascade of adaptive and maladaptive processes, such as systemic inflammation, endothelial dysfunction, and oxidative stress (3-5). Whilst the association of OSA with cardiovascular and metabolic co-morbidities is well recognised and described (6,7), its potential role in brain injury, leading to or aggravating existent neurological/psychiatric and neurodegenerative disorders, has only recently started receiving significant attention (5,8). Similarly, its potential role in the etiology of chronic kidney disease (CKD) and its association with cancer are not well understood. These co-morbidities and the intricate interplay between OSA and their pathogenesis are the main focus of this review.
OSA and impaired cognition
A variety of cognitive deficits, such as difficulties with attention, memory, executive functioning, and quality of life have an established relationship with OSA, alongside a number of psychiatric disorders in susceptible patients (8). Some of these impairments have been associated with corresponding structural changes (9,10). Continuous positive airway pressure (CPAP)—still the mainstay of OSA treatment—has been shown to at least partially reverse some of the cognitive impairments and associated neuroanatomical changes (8,11-13).
In addition, difficulty in forming functional interpersonal relationships, irritability, decreased work and school efficiency, and proneness to accidents, have all been documented in OSA patients (9,14,15). It has been proposed that OSA patients are two to thirteen times more likely to experience a driving-related traffic accident than the general population (16-19). Such accidents are more likely to occur in those with greater daytime sleepiness, but they are not necessarily related to sleepiness alone (16,20). In addition to cognitive and emotional deficits, the prevalence of OSA has been reported to be increased in several psychiatric disorders (21), with the strongest association seen with major depressive disorder (MDD), anxiety, and posttraumatic stress disorder (PTSD) (21-23).
The cognitive impairment seen in OSA tends to be more significant with increasing age (24-26), suggesting that older age groups are more susceptible to the effects of OSA on the brain (27), or that compensatory mechanisms that are perhaps present in younger people and help to recruit other areas of the brain to maintain performance, may no longer be as proficient (28). OSA is increasingly prevalent with age (29), although there tends to be less reported sleepiness in older patients. More recently, a link between Alzheimer’s disease (AD) and OSA has been suggested by a number of studies (30-33), with some authors arguing that dysregulated immunological mechanisms in the brain in OSA patients may contribute to the evolution of AD (9,34,35).
The results of a recent meta-analysis suggest that subjects with AD have an approximately five-fold likelihood of having concomitant OSA when compared with healthy controls (33). The causal relationship between untreated OSA and neurodegenerative processes is, however, unclear (5,9). The results of genome-wide analyses have shown that several genes that increase the risk for sporadic AD, also encode factors that regulate glial clearance of misfolded proteins and neuroinflammation (33,34).
It has been suggested that treatment of OSA, particularly in the early stages of AD, when patients are still largely independent, may decelerate the progression of dementia (36,37). CPAP appears to be partially effective in improving episodic verbal learning, memory, cognitive flexibility and mental processing speed in patients with co-morbid AD and OSA (36). Similarly, a study that analysed patients from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort found a significant association of self-reported sleep-disordered breathing with an earlier age of onset of mild cognitive impairment (31); mild cognitive impairment was observed approximately one decade earlier in sleep apnea patients, even when accounting for possible confounding factors. However, in those patients who used CPAP, this link appeared significantly attenuated, suggesting that use of CPAP may delay the progression of mild cognitive impairment (31).
Recent neuroimaging studies have suggested that sleep disruption and fragmentation provide a mechanism through which β-amyloid pathology contributes to hippocampus-dependent cognitive decline in the elderly (38,39). Inadequate sleep and significant sleep fragmentation present another important etiological aspect of OSA-induced brain injury and may contribute to the acceleration of ‘normal aging’ processes in patients with OSA (5).
Sleep fragmentation and deprivation
In previous studies that have deprived healthy individuals of sleep (40-42), sleep deprivation was associated with impairment in cortical and white matter regions, particularly those that likely underlie functions of attention and vigilance. It has been argued that sleep deprivation and fragmentation can independently lead to cognitive impairment, and in a similar pattern to what has been also associated with IH (43,44). Moreover, any significant sleep fragmentation likely disrupts restorative homeostatic synaptic processes in the brain and further interferes with memory consolidation and transformation, contingent on which sleep stages are affected (45,46). It also likely further precipitates neuroinflammatory processes and aggravates any predisposing immunological dysfunction (9,47). In a study assessing cognition in patients with untreated insomnia, narcolepsy and OSA versus controls, a shared impairment in alertness and selective attention, as well as decreased visual tracking, was identified (48). These findings were also associated with an increase in self-rated tiredness and daytime somnolence, and suggest a potential common pathological mechanism. Indeed, there is evidence that hypersomnolence itself (regardless of its cause) is an independent risk factor for cognitive impairment (49).
Residual cognitive impairment in patients who adhere well to their treatment with CPAP suggests a degree of irreversibility in some changes induced by OSA, and it likely points to importance of early recognition and induction of treatment (50,51). Equally, it is possible that CPAP as a treatment might not be a universal panacea for all stages of this chronic illness, and or that in certain susceptible patients CPAP treatment might need to be supplemented with other treatment modalities (e.g., antioxidants) in order to combat all aspects of neuroinflammation and oxidative stress injury induced by OSA (21).
IH
Hypoxia may affect the structure and function of the blood-brain barrier (52), leading to altered microvessel permeability and microregional oxidative processes, all of which could in the long term alter synaptic plasticity, and also contribute to cognitive impairment (3). Several mouse models have been used over the years to look at the effects of IH on the brain (53). Their results taken together suggest that IH leads to oxidative stress, inflammation, and regional and distant neuroanatomical and functional changes, depending on the severity, frequency and chronicity of the insult. IH-induced oxidative injury in the wake-promoting basal forebrain and brainstem in mice may lead to persistent hypersomnolence and associated cognitive impairment (54). Several studies comparing IH and sleep fragmentation in animal models have shown that similar brain regions are affected by both insults (24,43).
Apart from maladaptive processes, IH likely also starts cascade of adaptive homeostatic processes, such as ischaemic preconditioning, in all affected body systems, including the brain. Ischaemic pre-conditioning has been demonstrated in animal models in numerous body organs and systems (55,56). Moreover, it has been shown that ischemic insult in animals can lead to adult neurogenesis in neurogenic niches such as the dentate gyrus of the hippocampus and the olfactory bulb, and in some animals also in the striatum (57). Neurogliogenesis secondary to IH has also been postulated to occur in patients (58). In a recent study, hypertrophy of the hippocampi of patients with mild OSA was demonstrated, perhaps suggestive of such adaptive processes at work (59), whilst another study identified changes in creatine levels in the hippocampus in OSA patients, suggesting adjusted bioenergetics similar to those seen in ischaemic preconditioning (60).
Over the last several decades, numerous neuroimaging studies in patients with OSA have delineated a putative neurocircuitry fingerprint of sleep fragmentation and IH-induced brain injury (61). This wide circuitry suggests disconnection of the frontal regions [also see (21)], and the disruption of the (cerebello)-thalamocortical oscillator with involvement of the hippocampal formation.
Neuroanatomy of OSA
Historically, a number of voxel based morphometry (VBM) studies have investigated areas of the brain affected in patients with OSA with diverse regions being highlighted as more or less impacted (62-66). An early study assessing changes in patients with OSA reported diffuse changes in the frontal, temporal and parietal cortices, as well as in the anterior cingulate gyrus, hippocampus, and cerebellum (67). Subsequently, a combined VBM and PET imaging study demonstrated hypotrophic changes in wider circuitry, as well as a decrease in cortical metabolism despite no clinically overt cognitive deficits being present (64). This has been argued to suggest early processes likely leading to later clinically measurable cognitive changes. Later studies demonstrated the correlation between neuroimaging changes and cognitive deficits, including those in executive functioning and verbal memory (65,68).
In a recent comprehensive meta-analysis of functional and structural neuroimaging studies (10), structural atrophy and functional disturbances in the right basolateral amygdala, the hippocampus and the right central insula were demonstrated (Figures 1,2). Behavioural analyses of these regions suggested associated dysfunction of emotional, sensory and cognitive processes (Figure 3), and further highlighted a diffuse network within the bilateral anterior insula, posterior-medial frontal cortex and thalamus (Figure 4).
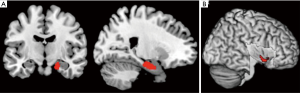
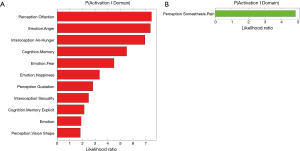
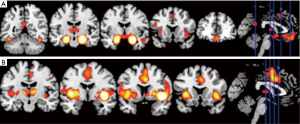
The importance of the amygdala as one of structures affected by the chronic process of OSA has been previously also shown in children (69). In children with OSA, greater neural recruitment of regions involved in cognitive control, conflict monitoring, and attentional allocation is required in order to perform at the same level as children without OSA (69). Of further note is that the amygdala has a recognised role in the etiology of anxiety disorders, MDD, and PTSD, potentially contributing to the increased prevalence of these psychopathologies in patients with OSA (10). The activation of the amygdala during emotional arousal enhances memory, in part by modulating plasticity in the hippocampus. It is possible that aberrant functional connectivity of amygdala and hippocampus in patients with OSA may further impact already dysfunctional intrinsic network activity in MDD, and that this may underlie some of the psychological disturbances in patients with OSA (10). In a similar vein, an aberrant connectivity between the hippocampus and the cerebellum, which has been previously reported in OSA patients, may lead to alterations in a distributed memory system for associative learning (59). On the other hand, an atypical engagement of the insula within the salience network appears to be common feature of OSA as well as of several neuropsychiatric disorders, including AD and MDD (10).
Functional MRI has also been used to show that OSA patients display decreased levels of activation in the frontal lobes (28,67), and an increased compensatory activation during memory and attention tasks in comparison to healthy controls (70,71). This increased activation was lost or ameliorated after CPAP treatment (71).
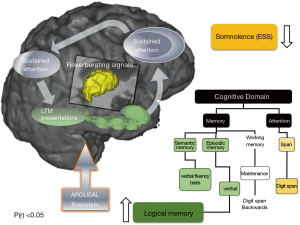
A beneficial effect of CPAP therapy in the treatment of cognitive deficits in subjects with OSA was suggested by results of a recent study by Canessa et al. (12). They showed that 3 months of CPAP treatment was associated with a significant increase in grey matter volume in the bilateral hippocampus and frontal structures, which correlated with an improvement in executive function, memory and attention (12). Similarly, in a recent study that used randomised controlled design, one month of CPAP treatment resulted in a partial recovery of episodic and working-memory capacity (Figure 4) (13). The observed thalamocortical changes were associated with changes in bilateral hippocampi and cerebellar cortices (13). These data suggest that a relatively short period of CPAP treatment may allow rudimentary neuroplastic changes to occur within targeted brain structures of patients with moderate to severe OSA, and that the structural changes observed provide a basic neurocognitive architectural scaffold for later reorganization that underscored some of the observed functional recovery in working and episodic memory (Figure 4). However, it has been also proposed that white matter changes observed in OSA patients may be refractory to treatment and may require longer treatment regimes (14), with a minimum of 12 months of CPAP treatment needed to reverse of some white matter changes recorded prior to commencement of therapy (11).
In conclusion, OSA-induced brain injury is an increasingly recognised entity and should be thought of as comprising a wide spectrum of related, if varied, neuropsychiatric presentations. Their variety and severity likely reflect idiosyncratic susceptibility of individual patients, as well as the interplay with the co-morbid impact of OSA on other body organs and systems, such as the cardiovascular system and the kidneys.
OSA and renal dysfunction
CKD is a highly prevalent disorder, associated with increased healthcare utilisation, hospitalisation and risk of death (72). Recognised contributory pathologies to its development include type 2 diabetes mellitus (T2DM) and hypertension, both common co-morbidities in patients with OSA. OSA appears to have a bi-directional relationship with CKD: the pathophysiological consequences of sleep disordered breathing have the potential to adversely affect kidney function, and lead to progression of established CKD (73), while altered fluid dynamics in advanced renal disease have an established detrimental effect on sleep breathing (74). Below we discuss the evidence in support of a role for OSA in the evolution of CKD, along with the impact of renal failure on OSA severity.
OSA as a risk factor for CKD
A potential relationship between OSA & CKD was first explored in 1988 (75) where 34 patients with OSA were reviewed and 6 found to have significant proteinuria on dipstick, 3 of which were seen to be in the nephrotic range. None of the healthy controls who were matched for age, sex and weight had proteinuria. Four OSA patients were followed for 4 years after starting CPAP therapy and a reduction in proteinuria was seen with treatment. However, it was some time before investigators began exploring this field in greater detail; more recent studies have suggested an increased prevalence of CKD in OSA cohorts, and have also evaluated the potential underlying mechanisms.
A significantly higher prevalence of CKD among OSA patients was identified in over 9,000 Japanese subjects: CKD was present in 30.5% of subjects with an AHI >5 events/hour, compared with 9.1% of the general population (adjusted OR 4.5) (76). In a carefully selected cohort with severe OSA, but without key CKD risk factors such as hypertension and diabetes, prevalence of CKD was found to be around 18% (77). Data from a retrospective study of 161 non-obese Japanese patients with stage 3–4 CKD showed a relative fourfold rate of decline in eGFR in subjects with a 4% ODI ≥15 (78), while an adjusted OR of 2.89 for accelerated loss of renal function was observed among subjects with nocturnal hypoxemia (defined as spending ≥12% of study time with an SpO2 <90%) in another retrospective cohort study involving 858 subjects referred to a Canadian sleep unit (79). Perhaps the best population-level evidence in this fields comes from a longitudinal study of 4674 Taiwanese patients with sleep disordered breathing who were compared with 23,370 age- and sex-matched controls; following adjustment for relevant confounding diagnosis and demographic factors, subjects with sleep apnea had a 1.94-fold risk of developing CKD and a 2.2-fold increase in incidence of end stage renal disease (ESRD) over a 5-year period (80).
These are not uniform findings. A retrospective review evaluating kidney function in 634 Turkish patients with OSA and 62 healthy controls, compared estimated glomerular filtration rate (eGFR) in OSA patients with and without metabolic syndrome and LVH (81). Subjects with LVH and metabolic syndrome had a significantly lower eGFR when compared with OSA patients without these co-morbidities or with healthy controls. However, no significant differences were seen in eGFR in OSA patients without LVH or metabolic syndrome compared to controls, suggesting that indirect effects of OSA such as hypertension, precipitate renal changes and eventually dysfunction. Similarly, in a cross-sectional analysis of 8,112 participants in the multi-national ESADA study, diabetes and hypertension were independent predictors of mild-moderate renal impairment, while AHI and ODI were not (82).
Pathophysiology of CKD in OSA patients
Renal dysfunction in OSA patients may arise via traditional risk factors for CKD associated with OSA, or it may be the result of the characteristic pathophysiological consequences of sleep disordered breathing, such as increased sympathetic output, oxidative stress, systemic inflammation, and nocturnal hypoxemia.
Conventional CKD risk factors and OSA
The relationship between OSA and cardio-metabolic disorders with established links to the evolution of CKD is the subject of an extensive and growing evidence base. OSA is an independent risk factor for the development of hypertension, and appears to have independent associations with the development of atherosclerosis and diabetes mellitus prevalence and control (83-85). The potential causative role of OSA in the development of these important co-morbidities is the subject of recent dedicated reviews in the Journal of Thoracic Disease (7,86), and will not be discussed in detail here. Notably, OSA may hasten the development of diabetic nephropathy (DN), as evidenced by an increased prevalence of OSA in subjects with DN, and an increased rate of progression of CKD in OSA patients, in a cohort of 224 type 2 diabetes followed over 2.5 years (87).
Another potential confounding factor in studies assessing the relationship between kidney disease and OSA is the effect of obesity on renal function. Obesity is an established risk factor for the presence of CKD: a meta-analysis of 19 cohorts evaluating the relationship between increasing body mass and renal function identified a pooled relative risk of 1.83 (95% CI 1.57–2.13) for CKD among subjects with a BMI ≥30 kg/m2 when compared with lean cohorts, following adjustment for relevant confounding factors (88). A case control study comparing renal biopsies in 95 morbidly obese patients undergoing bariatric surgery with 40 control subjects identified an independent relationship between BMI and likelihood of glomerular lesions (podocyte hypertrophy, increased mesangial cellularity or increased extracellular matrix) (89). Other investigators have found obesity to be associated with glomerular hypertrophy, basement membrane thickening, and overt proteinuria (90,91).
OSA as a causative factor in CKD
Pathways through which sleep disordered breathing may exert a direct impact on renal function include alterations in glomerular perfusion mediated by OSA-related changes in sympathetic tone, neurohumeral output, and endothelial function, alongside tubulointerstitial hypoxia due to repetitive nocturnal fluctuations in oxygen tension (73). Low grade systemic inflammation promotes endothelial dysfunction and subsequent atherosclerosis, and occurs in OSA patients as a direct result of nocturnal IH (92). Similarly, IH promotes the generation of free oxygen radicals, with the consequent oxidative stress exerting a detrimental effect on the vascular endothelium (93), while apneic events also lead to increased sympathetic outflow, leading to profound alterations in vascular tone (94). In a study comparing healthy controls with newly diagnosed patients with moderate to severe OSA, but free of cardiovascular risk factors, the OSA patients showed evidence of endothelial dysfunction, impaired renal vasodilation and reduced endothelial nitric oxide synthase expression, despite a lack of overt signs of end organ disease. This suggests that OSA in itself is an independent risk factor for clinically occult renal endothelial damage which is not clinically detectible, which may then proceed to overt renal dysfunction (95).
An important potential mechanism mediating the development of CKD in OSA patients is activation of the renin-angiotensin system (RAS). Alongside increasing systemic blood pressure, disproportionate RAS activation leads to glomerular hyperperfusion and subsequent glomerulosclerosis. A number of studies have suggested that OSA may lead to systemic RAS activation, with data from rodent models suggesting that IH may play a key role (96). An elegant, sham controlled, cross-over study examined systemic BP changes in nine healthy subjects exposed variously to sham IH, IH with placebo, and IH with the type I angiotensin II receptor antagonist losartan. Exposure to IH, but not sham IH, lead to a significant increase in BP, an increase that was entirely abrogated by losartan, suggesting a key role for IH in causing alterations in RAS activation.
Recently, reduced renovascular sensitivity in response to a 60-minute angiotensin II infusion was observed in OSA patients with severe nocturnal hypoxemia when compared with milder OSA patients and non-apneic, obese controls (97). No changes were observed in BP or in circulating markers of RAS activation, suggesting a specific direct impact of IH on the kidney. The effects of IH on RAS activity may be mediated via changes in sympathetic tone, with renovascular denervation in a pig model of OSA preventing apnea-related changes in RAS activation (98).
Effect of CPAP on renal function
Were OSA contributing to the development of CKD, the use of nocturnal CPAP therapy might be expected to lead to measureable improvements in indices of renal RAS activation and renal function. There is evidence that compliance with CPAP therapy in OSA patients with known renal disease can slow the progression of CKD—a retrospective study of 42 patients with OSA and stage 3–5 CKD found that decline in eGFR over 2 years was significantly more precipitous in subjects who were poorly compliant with CPAP therapy (99). A week of nocturnal CPAP reduced glomerular hyperfiltration in 24 men with moderately severe OSA (100), while two studies involving ultrasonographic evaluation of the renal circulation in OSA subjects, showed a significant improvement in intra-renal haemodynamics after initiation of CPAP (101,102). These changes may be attributable to alterations in RAS activity following commencement of CPAP therapy; Nicholl et al measured changes in renal plasma flow response to infused angiotensin II as an indirect measure of the intrarenal RAS before and after one month of CPAP therapy in 20 patients, finding that CPAP use was associated with reduced circulating aldosterone levels and reduced overall intra-renal RAS activity (103). Overall, a growing body of evidence suggests that OSA may directly and indirectly contribute to the decline of kidney function, and that CPAP therapy may at least partially ameliorate this decline, although further interventional studies examining the effect of CPAP on longitudinal outcomes are required.
CKD as a contributor to OSA severity
Sleep disordered breathing is common in CKD cohorts, and its prevalence increases as renal function deteriorates, with a study of 254 subjects representing the full spectrum of renal function finding that 41% of CKD patients and 57% of ESRD patients had significant sleep apnea, as defined by an RDI ≥15, compared to 27% of those with an eGFR ≥60 mL/min/kg2 (104). In OSA, a combination of anatomical features, changes in ventilatory control, and modifiable environmental factors such as obesity lead to an increased propensity for upper airways collapse during sleep (105). In advanced CKD & ESRD these mechanisms also apply, but there is increasing evidence that peripheral volume overload and rostral fluid shift - whereby fluid shifts from the legs and torso towards the head and neck while recumbent—may be important additional contributors (74).
Measurement of upper airway dimensions with acoustic pharyngometry shows that ESRD patients have reduced pharyngeal area when compared with subjects with normal renal function (106). Inducing a transient state of volume overload with the infusion of a high volume of isotonic saline solution during sleep in healthy men >40 years old leads to a marked increase in AHI, occurring in parallel with an increase of neck circumference (107). In ESRD patients, the extent of rostral fluid shift, as measured by changes in leg fluid volume, correlates with OSA severity indices, a relationship which survives adjustment for confounding variables (108). Pulmonary congestion is common in dialysis patients, even when relatively asymptomatic, and may lead to altered chemosensitivity, loop gain and reduced ventilator stability, potentially further increasing the severity of sleep disordered breathing (109,110).
While ESRD may also contribute to sleep disordered breathing via uremic destabilisation of central respiratory control (109), data from studies of varying dialysis strategies suggest that alterations in extravascular fluid volume may be of more relevance to OSA severity. Conventional hemodialysis (HD) does not appear to significantly abrogate the degree of OSA present, but switching ESRD patients to a nocturnal HD strategy is associated with marked improvements in sleep breathing (111), while renal transplantation may lead to resolution of sleep apnea (112). That volume removal, rather than metabolic changes occurring post-HD, is the key factor here is suggested by a recent elegant study of changes in sleep breathing in 15 HD-dependent patients with moderate-severe OSA, who underwent PSG before and after aggressive fluid removal by ultra-filtration (UF) (113). Following UF, AHI was reduced by 36%, and a significant improvement in objective sleep quality was observed, with these improvements correlating with reductions in measures of peripheral volume overload.
It is certainly clear that renal dysfunction is associated with OSA, and that OSA is found in higher incidence in CKD patients. However, as with all research into patients with OSA, there are many potential confounding co-morbidities which are difficult to account for when determining underlying mechanisms. Further larger studies are needed to assess whether OSA and IH directly lead to CKD, and therefore whether more measures need to be taken to monitor and treat these patients earlier to prevent this end organ damage, and whether all patients with CKD and ESRD should be screened for OSA and treated as early as possible to prevent further decline.
OSA and cancer
A decade of research into the cardiovascular consequences of sleep disordered breathing has shown OSA to lead to chronic systemic inflammation, oxidative stress, and immune dysregulation. Alongside recurrent nocturnal hypoxemia and local tissue hypoxia, these factors contribute to the development of an oncogenic milieu. A recognition of this has led to a recent spate of studies exploring how sleep apnea may influence cancer incidence and outcomes. Unusually, the evidence base in this field started with initial proof of concept studies in animal models of IH, before moving on to clinical studies confirming a potential causative role for OSA in carcinogenesis, but for the purposes of this brief review, longitudinal population studies shall be discussed first, before moving on to animal and mechanistic data.
Cancer incidence and outcomes in OSA populations
A number of community- and sleep laboratory-based population studies have found an association between severity of sleep disordered breathing and cancer incidence. In a multicentre sleep clinic cohort involving 4,910 patients followed for a median of 4.5 years, incidence of cancer was assessed across tertiles of nocturnal hypoxemia, as measured by the cumulative sleep time with oxygen saturations <90% (ct90) (114). Compared to subjects in the lowest tertile (ct90 <1.2%), those in the highest (ct90 >12%) had an adjusted HR of 2.33 for developing malignancy. This relationship was strongest in participants under the age of 65 yrs. A smaller study of 400 community based Australian subjects found that moderate-severe OSA (RDI >15) predicted a 2.5-fold risk of incident cancer over a 20-year follow-up (115). Data from an administrative claims database in Taiwan examined the relationship of OSA with organ-specific cancer risk. Among 23,055 OSA patients, the risk of central nervous system (CNS) malignancy was 1.5 times that of age- and gender-matched non-apneic controls over a 2-year period, with the greatest risk seen in subjects with both OSA and insomnia (HR 2.20) (116). A five year follow up of 846 women with OSA found a two-fold risk of breast cancer when compared to age-matched controls in a cohort derived from a subset of the same database (117).
Not all investigators have found a link between OSA and cancer risk, however. Over a mean follow-up of 13 years, no overall association between symptoms of sleep apnea and subsequent cancer risk was seen in 8,783 participants in the Copenhagen City Heart Study, although there was a relationship between subjective sleepiness and incident malignancy in younger subjects (118). While this study was limited by its lack of objective measurements of sleep disordered breathing, a large Canadian study of nearly 10,000 patients undergoing in-laboratory PSG similarly found that any association between OSA severity and cancer risk appeared to be attributable to conventional risk factors, such as age, smoking and obesity (119). Nonetheless, even within this latter cohort, sub-group analyses suggested a link between severity of nocturnal hypoxemia and smoking-related cancer incidence.
Fewer studies have evaluated the relationship between OSA and risk of cancer death. What data is available suggests that subjects with more severe sleep apnea are more likely to die of cancer than those with mild or no sleep disordered breathing. In a 22-year follow-up of 1,522 participants in the community-based Wisconsin Sleep Cohort Study, a dose-response relationship between OSA severity and cancer-specific mortality was observed, with severe OSA conferring a nearly five-fold risk of death from cancer (adjusted RR 4.8) (120). This relationship was particularly strong in those with the most severe degree of nocturnal hypoxemia, and in non-obese subjects. Participants in the Australian Busselton Health Study had a 3.4-fold risk of cancer death if they had moderate-severe OSA (115), while data from Spanish sleep laboratory patients followed for an average of 4.5 years demonstrated an increased risk of death with increasing degrees of nocturnal hypoxemia, particularly in patients <65 years of age (121). A summary of population and clinical studies of cancer incidence and death in OSA patients is presented in Table 1.
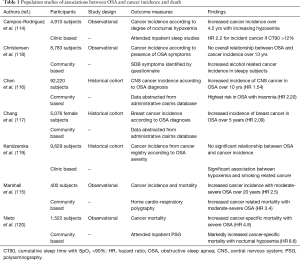
Full table
Cancer and OSA in animal models and clinical studies
Initial studies evaluating interactions between OSA and cancer were largely performed in animal models of IH. In a ground-breaking series of studies, Almendros et al. showed that IH had a clear detrimental effect in mice inoculated with melanoma cells. In animals where melanoma was induced by injection of malignant cells into the flank, exposure to 17 days of IH for 6 hours/day was associated with an increased rate of tumour growth, which occurred in parallel with increased circulating levels of vasoactive endothelial growth factor (VEGF) (122). Moreover, 30 days of IH lead to an increased number and volume of pulmonary metastases when compared to control mice (123). A similar study protocol using epithelial lung tumour cells also showed increased rates of cancer progression in IH-exposed animals (124), while other OSA-related factors, such as sleep fragmentation, have similarly been shown to accelerate tumour growth in murine IH models (125).
There is at present a paucity of clinical studies examining the effect of OSA on cancer progression. In a multicentre observational studies of 82 patients diagnosed with cutaneous melanoma, OSA severity indices independently predicted tumour growth rate, along with other indicators of aggressiveness. These findings need to be confirmed in other clinic populations, and it is as yet unknown if CPAP therapy could have a modifying role in cancer outcomes.
Mechanisms of carcinogenesis in OSA
Systemic inflammation and oxidative stress
There is a longstanding recognition that any disorder associated with chronic local or systemic inflammation may be associated with an increased risk of cancer development, an effect potentially mediated or amplified by the generation of reactive oxygen and reactive nitrogen species, with associated DNA damage and impaired DNA repair (126). OSA and associated IH have been demonstrated to independently contribute to the development of low-grade systemic inflammation (6), with increased expression of NF-κB dependent cytokines in both clinical subjects (127) and in vitro models of disease (128). Similarly, a significant evidence base supports the notion that OSA is an oxidative stress disorder, with reactive oxygen and nitrogen species likely formed at least partially due to IH-mediated mitochondrial dysfunction (93). Nocturnal CPAP therapy may lead to an abrogation of both systemic inflammation and oxidative stress, suggesting that appropriate treatment may obviate any pro-carcinogenic effect of OSA.
Hypoxemia and tissue hypoxia
Local tissue hypoxia induces stability of hypoxia-inducible factor-1α (HIF-1α), which under normoxic conditions is degraded by a series proline hydroxylases (129). In general terms, this can be considered an adaptive process, with HIF acting as a master regulator of cellular responses to decreased oxygen supply or increased oxygen consumption, but it appears to play a key maladaptive role in tumour biology (130). HIF-1 dependent-genes promote cell proliferation and angiogenesis, and facilitate metabolic adaptation to the hypoxic environment, thereby promoting tumour survival and progression (130). While OSA leads to intermittent hypoxemia, it is unclear if this translates into intermittent or sustained hypoxia at a tissue level. Rodent data would suggest that severe chronic IH may lead to fluctuating oxygen levels in hepatic tissue, but sustained hypoxia in adipose tissue (131). Human data are lacking, but sustained tissue hypoxia leads to a robust increase in expression of HIF-dependent genes, perhaps promoting carcinogenesis. Moreover, hypoxia is a pro-inflammatory stimulus, with HIF and NF-κB interacting to drive increased inflammatory cytokine expression (132,133).
Sleep fragmentation and increased sympathetic drive
Generally considered to be mediators of the adverse cardiovascular consequences of sleep disordered breathing, sleep fragmentation and increased sympathetic tone induced by apneic events may also contribute to cancer development. As mentioned above, animal models of sleep fragmentation lead to increased tumour progression; the potential immunological mechanisms behind this will be discussed below (125). Sleep disruption and restriction seen in shift workers are also associated with an increased risk of cancer incidence (134), but specific data examining the relationship between sleep fragmentation per se and cancer are lacking (135). Increased adrenergic signalling may also promote cancer survival and growth, although there is again a paucity of evidence specific to OSA patients in this regard (135).
Alterations in immune function
A key effector cell in cancer biology is the macrophage, and robust evidence shows that an increase in tumour associated macrophages (TAMs), particularly those with an anti-inflammatory M2 phenotype, is associated with worse outcomes in a variety of cancers (136). TAMs appear to promote angiogenesis, and promote tumour invasion and metastatic capacity. In a murine model of IH, TAMs showed a shift in polarity to a pro-tumoural M2 phenotype, and TAMs explanted from IH-exposed mice enhanced proliferation and invasiveness of pulmonary epithelial cancer cells in vitro (124,137).
Data examining tumour-specific immune function in OSA patients also suggests that sleep disordered breathing may contribute to a reduction in innate anti-tumoural responses. Genome sequencing in circulating leucocytes harvested from treatment-naïve patient with severe OSA revealed an upregulation in pro-neoplastic gene sets (138), and a subsequent downregulation in expression of these genes following approximately one month of CPAP therapy.
Another important effector cell in host responses to tumour development is the invariant natural killer T cell (iNKT). These are potent immunomodulatory cells which play a key role in conducting both innate and adaptive immune responses, and which have been demonstrated to direct anti-tumour responses and to directly lyse tumour cells (139). A recent novel study found a marked reduction in circulating iNKT cells in patients with severe OSA, correlating directly with indices of OSA severity independently of the confounding effects of obesity (140). Furthermore, a year of CPAP therapy lead to a partial restoration in iNKT numbers, and a series of in vitro models showed that hypoxia lead to a reduction in their ability to lyse tumour cells.
Overall, circumstantial, epidemiological and animal-based data support a role for OSA in promoting carcinogenesis and cancer progression. However, further mechanistic studies are required to better understand the underlying molecular alterations that underpin this association, and large scale, well designed trials of CPAP therapy will be required to demonstrate that OSA constitutes a modifiable risk factor for cancer development.
Conclusions
The relationship between OSA and cardiovascular morbidity is well established, and it seems increasingly likely that sleep disordered breathing also promotes the development of metabolic disease. However, the pathophysiological effects of OSA appear to extend beyond the vasculature and endocrine function, with a growing evidence base suggesting that it is of significant relevance in neurocognitive functioning, the development of CKD, and cancer risk and outcomes. As emphasised above, all three of these areas represent emerging fields, and ongoing high quality studies are required to further our understanding of the processes concerned.
Acknowledgements
Funding: This work was supported by the Wellcome Trust (103952/Z/14/Z).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 2015;7:1311-22. [PubMed]
- Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997;20:705-6. [PubMed]
- Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Med Rev 2015;20:27-45. [Crossref] [PubMed]
- Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 2015:15015.
- Rosenzweig I, Glasser M, Polsek D, et al. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med 2015;3:404-14. [Crossref] [PubMed]
- Kent BD, Ryan S, McNicholas WT. Obstructive sleep apnea and inflammation: relationship to cardiovascular co-morbidity. Respir Physiol Neurobiol 2011;178:475-81. [Crossref] [PubMed]
- Kent BD, McNicholas WT, Ryan S. Insulin resistance, glucose intolerance and diabetes mellitus in obstructive sleep apnoea. J Thorac Dis 2015;7:1343-57. [PubMed]
- Kylstra WA, Aaronson JA, Hofman WF, et al. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev 2013;17:341-7. [Crossref] [PubMed]
- Rosenzweig I, Glasser M, Polsek D, et al. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med 2015;3:404-14. [Crossref] [PubMed]
- Tahmasian M, Rosenzweig I, Eickhoff SB, et al. Structural and functional neural adaptations in obstructive sleep apnea: An activation likelihood estimation meta-analysis. Neurosci Biobehav Rev 2016;65:142-56. [Crossref] [PubMed]
- Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014;37:1465-75. [PubMed]
- Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med 2011;183:1419-26. [Crossref] [PubMed]
- Rosenzweig I, Glasser M, Crum WR, et al. Changes in Neurocognitive Architecture in Patients with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure. EBioMedicine 2016;7:221-9. [Crossref] [PubMed]
- Kryger MH, Roth TI, Dement WC. editors. Principles and practice of sleep medicine. 5th ed. Philadelphia, PA: Saunders/Elsevier, 2011.
- Twigg GL, Papaioannou I, Jackson M, et al. Obstructive sleep apnea syndrome is associated with deficits in verbal but not visual memory. Am J Respir Crit Care Med 2010;182:98-103. [Crossref] [PubMed]
- Ellen RL, Marshall SC, Palayew M, et al. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med 2006;2:193-200. [PubMed]
- Khazaie H, Maroufi A. Obstructive sleep apnea syndrome; a neglected cause of traffic collision among Iranian public transport drivers. J Inj Violence Res 2014;6:99. [PubMed]
- Karimi M, Hedner J, Häbel H, et al. Sleep apnea-related risk of motor vehicle accidents is reduced by continuous positive airway pressure: Swedish Traffic Accident Registry data. Sleep 2015;38:341-9. [PubMed]
- Karimi M, Hedner J, Zou D, et al. Attention deficits detected in cognitive tests differentiate between sleep apnea patients with or without a motor vehicle accident. Sleep Med 2015;16:528-33. [Crossref] [PubMed]
- Weaver TE, George CF. Cognition and performance in patients with obstructive sleep apnea. In: Kryger MH, Roth TI, Dement WC. editors. Principles and practice of sleep medcine. 5th edition. St Louis (MO): Elsevier Saunders, 2011:1194-206.
- Rosenzweig I, Weaver T, Morrell MJ. Obstructive Sleep Apnea and the Central Nervous System: Neural Adaptive Processes, Cognition and Performance. In: Kryger MH, Roth T, Dement MC. editors. Principles and practice of sleep medicine. Sixth edition. Philadelphia, PA: Elsevier, 2017.
- Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med 2015;11:165-75. [PubMed]
- Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep 2005;28:1405-11. [PubMed]
- Sforza E, Roche F. Sleep apnea syndrome and cognition. Front Neurol 2012;3:87. [Crossref] [PubMed]
- Mathieu A, Mazza S, Decary A, et al. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep Med 2008;9:112-20. [Crossref] [PubMed]
- Alchanatis M, Zias N, Deligiorgis N, et al. Comparison of cognitive performance among different age groups in patients with obstructive sleep apnea. Sleep Breath 2008;12:17-24. [Crossref] [PubMed]
- Grigg-Damberger M, Ralls F. Cognitive dysfunction and obstructive sleep apnea: from cradle to tomb. Curr Opin Pulm Med 2012;18:580-7. [Crossref] [PubMed]
- Ayalon L, Ancoli-Israel S, Drummond SP. Obstructive sleep apnea and age: a double insult to brain function? Am J Respir Crit Care Med 2010;182:413-9. [Crossref] [PubMed]
- McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med 2014;2:804-12. [Crossref] [PubMed]
- Pan W, Kastin AJ. Can sleep apnea cause Alzheimer's disease? Neurosci Biobehav Rev 2014;47:656-69. [Crossref] [PubMed]
- Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015;84:1964-71. [Crossref] [PubMed]
- Osorio RS, Ayappa I, Mantua J, et al. Interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer's disease in cognitively normal elderly individuals. Neurobiol Aging 2014;35:1318-24. [Crossref] [PubMed]
- Emamian F, Khazaie H, Tahmasian M, et al. The Association Between Obstructive Sleep Apnea and Alzheimer's Disease: A Meta-Analysis Perspective. Front Aging Neurosci 2016;8:78. [Crossref] [PubMed]
- Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol 2015;14:388-405. [Crossref] [PubMed]
- Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer's disease. Nat Immunol 2015;16:229-36. [Crossref] [PubMed]
- Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc 2008;56:2076-81. [Crossref] [PubMed]
- Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer's disease. J Neurol Neurosurg Psychiatry 2014;85:1405-8. [Crossref] [PubMed]
- Mander BA, Marks SM, Vogel JW, et al. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 2015;18:1051-7. [Crossref] [PubMed]
- Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci 2013;16:357-64. [Crossref] [PubMed]
- Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep 2007;7:161-6. [Crossref] [PubMed]
- Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res 2010;185:105-29. [Crossref] [PubMed]
- Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res 2011;190:53-68. [Crossref] [PubMed]
- Nair D, Dayyat EA, Zhang SX, et al. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One 2011;6:e19847. [Crossref] [PubMed]
- Daurat A, Foret J, Bret-Dibat JL, et al. Spatial and temporal memories are affected by sleep fragmentation in obstructive sleep apnea syndrome. J Clin Exp Neuropsychol 2008;30:91-101. [Crossref] [PubMed]
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 2002;11:1-16. [Crossref] [PubMed]
- Tononi G, Cirelli C. Time to be SHY? Some comments on sleep and synaptic homeostasis. Neural Plast 2012;2012:415250.
- Besedovsky L, Born J. Sleep, don't sneeze: longer sleep reduces the risk of catching a cold. Sleep 2015;38:1341-2. [PubMed]
- Schneider C, Fulda S, Schulz H. Daytime variation in performance and tiredness/sleepiness ratings in patients with insomnia, narcolepsy, sleep apnea and normal controls. J Sleep Res 2004;13:373-83. [Crossref] [PubMed]
- Verstraeten E, Cluydts R, Pevernagie D, et al. Executive function in sleep apnea: controlling for attentional capacity in assessing executive attention. Sleep 2004;27:685-93. [PubMed]
- Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain Res Bull 2003;61:87-92. [Crossref] [PubMed]
- Lau EY, Eskes GA, Morrison DL, et al. Executive function in patients with obstructive sleep apnea treated with continuous positive airway pressure. J Int Neuropsychol Soc 2010;16:1077-88. [Crossref] [PubMed]
- Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier. Sleep Med Rev 2014;18:35-48. [Crossref] [PubMed]
- Gozal D. Effects of intermittent hypoxia on neurological function. NY: Human Press, 2009.
- Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 2004;27:194-201. [PubMed]
- Tsai YW, Yang YR, Wang PS, et al. Intermittent hypoxia after transient focal ischemia induces hippocampal neurogenesis and c-Fos expression and reverses spatial memory deficits in rats. PLoS One 2011;6:e24001. [Crossref] [PubMed]
- Lavie L, Lavie P. Ischemic preconditioning as a possible explanation for the age decline relative mortality in sleep apnea. Med Hypotheses 2006;66:1069-73. [Crossref] [PubMed]
- Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab 2006;26:1-20. [Crossref] [PubMed]
- Nanduri J, Yuan G, Kumar GK, et al. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol 2008;164:277-81. [Crossref] [PubMed]
- Rosenzweig I, Kempton MJ, Crum WR, et al. Hippocampal hypertrophy and sleep apnea: a role for the ischemic preconditioning? PLoS One 2013;8:e83173. [Crossref] [PubMed]
- Bartlett DJ, Rae C, Thompson CH, et al. Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep Med 2004;5:593-6. [Crossref] [PubMed]
- Rosenzweig I, Williams SC, Morrell MJ. The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Curr Opin Pulm Med 2014;20:565-71. [Crossref] [PubMed]
- Morrell MJ, McRobbie DW, Quest RA, et al. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med 2003;4:451-4. [Crossref] [PubMed]
- O'Donoghue FJ, Briellmann RS, Rochford PD, et al. Cerebral structural changes in severe obstructive sleep apnea. Am J Respir Crit Care Med 2005;171:1185-90. [Crossref] [PubMed]
- Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res 2009;18:36-48. [Crossref] [PubMed]
- Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage 2011;54:787-93. [Crossref] [PubMed]
- Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 2002;166:1382-7. [Crossref] [PubMed]
- Thomas RJ, Rosen BR, Stern CE, et al. Functional imaging of working memory in obstructive sleep-disordered breathing. J Appl Physiol (1985) 2005;98:2226-34. [Crossref] [PubMed]
- Zhang Q, Wang D, Qin W, et al. Altered resting-state brain activity in obstructive sleep apnea. Sleep 2013;36:651-659B. [PubMed]
- Kheirandish-Gozal L, Yoder K, Kulkarni R, et al. Preliminary functional MRI neural correlates of executive functioning and empathy in children with obstructive sleep apnea. Sleep 2014;37:587-92. [PubMed]
- Archbold KH, Borghesani PR, Mahurin RK, et al. Neural activation patterns during working memory tasks and OSA disease severity: preliminary findings. J Clin Sleep Med 2009;5:21-7. [PubMed]
- Castronovo V, Canessa N, Strambi LF, et al. Brain activation changes before and after PAP treatment in obstructive sleep apnea. Sleep 2009;32:1161-72. [PubMed]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. [Crossref] [PubMed]
- Hanly PJ, Ahmed SB. Sleep apnea and the kidney: is sleep apnea a risk factor for chronic kidney disease? Chest 2014;146:1114-22. [Crossref] [PubMed]
- Kent BD, Steier J. A brief history of fluid and sleep. Am J Respir Crit Care Med 2015;191:1219-20. [Crossref] [PubMed]
- Chaudhary BA, Sklar AH, Chaudhary TK, et al. Sleep apnea, proteinuria, and nephrotic syndrome. Sleep 1988;11:69-74. [PubMed]
- Iseki K, Tohyama K, Matsumoto T, et al. High Prevalence of chronic kidney disease among patients with sleep related breathing disorder (SRBD). Hypertens Res 2008;31:249-55. [Crossref] [PubMed]
- Chou YT, Lee PH, Yang CT, et al. Obstructive sleep apnea: a stand-alone risk factor for chronic kidney disease. Nephrol Dial Transplant 2011;26:2244-50. [Crossref] [PubMed]
- Sakaguchi Y, Hatta T, Hayashi T, et al. Association of nocturnal hypoxemia with progression of CKD. Clin J Am Soc Nephrol 2013;8:1502-7. [Crossref] [PubMed]
- Ahmed SB, Ronksley PE, Hemmelgarn BR, et al. Nocturnal hypoxia and loss of kidney function. PLoS One 2011;6:e19029. [Crossref] [PubMed]
- Lee YC, Hung SY, Wang HK, et al. Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. Sleep 2015;38:213-21. [PubMed]
- Uyar M, Davutoğlu V, Gündoğdu N, et al. Renal functions in obstructive sleep apnea patients. Sleep Breath 2016;20:191-5. [Crossref] [PubMed]
- Marrone O, Battaglia S, Bonsignore MR. Relationship between mild to moderate renal dysfunction and obstructive sleep apnea: Data from the European sleep apnea database. Eur Respir J 2013;42:4037.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378-84. [Crossref] [PubMed]
- Mooe T, Franklin KA, Holmstrom K, et al. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med 2001;164:1910-3. [Crossref] [PubMed]
- Kent BD, Grote L, Ryan S, et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest 2014;146:982-90. [Crossref] [PubMed]
- Garvey JF, Pengo MF, Drakatos P, et al. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis 2015;7:920-9. [PubMed]
- Tahrani AA, Ali A, Raymond NT, et al. Obstructive sleep apnea and diabetic nephropathy: a cohort study. Diabetes Care 2013;36:3718-25. [Crossref] [PubMed]
- Wang Y, Chen X, Song Y, et al. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 2008;73:19-33. [Crossref] [PubMed]
- Serra A, Romero R, Lopez D, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int 2008;73:947-55. [Crossref] [PubMed]
- Kato S, Nazneen A, Nakashima Y, et al. Pathological influence of obesity on renal structural changes in chronic kidney disease. Clin Exp Nephrol 2009;13:332-40. [Crossref] [PubMed]
- Goumenos DS, Kawar B, El Nahas M, et al. Early histological changes in the kidney of people with morbid obesity. Nephrol Dial Transplant 2009;24:3732-8. [Crossref] [PubMed]
- Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax 2009;64:631-6. [PubMed]
- Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J 2009;33:1467-84. [Crossref] [PubMed]
- Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2011;184:1192-9. [Crossref] [PubMed]
- Bruno RM, Rossi L, Fabbrini M, et al. Renal vasodilating capacity and endothelial function are impaired in patients with obstructive sleep apnea syndrome and no traditional cardiovascular risk factors. J Hypertens 2013;31:1456-64; discussion 1464. [Crossref] [PubMed]
- Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 1999;34:309-14. [Crossref] [PubMed]
- Zalucky AA, Nicholl DD, Hanly PJ, et al. Nocturnal Hypoxemia Severity and Renin-Angiotensin System Activity in Obstructive Sleep Apnea. Am J Respir Crit Care Med 2015;192:873-80. [Crossref] [PubMed]
- Linz D, Mahfoud F, Linz B, et al. Effect of obstructive respiratory events on blood pressure and renal perfusion in a pig model for sleep apnea. Am J Hypertens 2014;27:1293-300. [Crossref] [PubMed]
- Puckrin R, Iqbal S, Zidulka A, et al. Renoprotective effects of continuous positive airway pressure in chronic kidney disease patients with sleep apnea. Int Urol Nephrol 2015;47:1839-45. [Crossref] [PubMed]
- Kinebuchi S, Kazama JJ, Satoh M, et al. Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin Sci (Lond) 2004;107:317-22. [Crossref] [PubMed]
- Sardo L, Palange P, Di Mario F, et al. Intrarenal hemodynamic and oxidative stress in patients with obstructive sleep apnea syndrome. Sleep Breath 2015;19:1205-12. [Crossref] [PubMed]
- Buchner NJ, Wissing KR, Stegbauer J, et al. The renal resistance index is increased in mild-to-moderate obstructive sleep apnoea and is reduced under continuous positive airway pressure. Nephrol Dial Transplant 2011;26:914-20. [Crossref] [PubMed]
- Nicholl DD, Hanly PJ, Poulin MJ, et al. Evaluation of continuous positive airway pressure therapy on renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med 2014;190:572-80. [Crossref] [PubMed]
- Nicholl DD, Ahmed SB, Loewen AH, et al. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest 2012;141:1422-30. [Crossref] [PubMed]
- Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383:736-47. [Crossref] [PubMed]
- Beecroft JM, Hoffstein V, Pierratos A, et al. Pharyngeal narrowing in end-stage renal disease: implications for obstructive sleep apnoea. Eur Respir J 2007;30:965-71. [Crossref] [PubMed]
- Yadollahi A, Gabriel JM, White LH, et al. A randomized, double crossover study to investigate the influence of saline infusion on sleep apnea severity in men. Sleep 2014;37:1699-705. [PubMed]
- Elias RM, Bradley TD, Kasai T, et al. Rostral overnight fluid shift in end-stage renal disease: relationship with obstructive sleep apnea. Nephrol Dial Transplant 2012;27:1569-73. [Crossref] [PubMed]
- Roumelioti ME, Brown LK, Unruh ML. The Relationship Between Volume Overload in End-Stage Renal Disease and Obstructive Sleep Apnea. Semin Dial 2015;28:508-13. [Crossref] [PubMed]
- Salloum A, Rowley JA, Mateika JH, et al. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 2010;181:189-93. [Crossref] [PubMed]
- Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med 2001;344:102-7. [Crossref] [PubMed]
- Auckley DH, Schmidt-Nowara W, Brown LK. Reversal of sleep apnea hypopnea syndrome in end-stage renal disease after kidney transplantation. Am J Kidney Dis 1999;34:739-44. [Crossref] [PubMed]
- Lyons OD, Chan CT, Yadollahi A, et al. Effect of ultrafiltration on sleep apnea and sleep structure in patients with end-stage renal disease. Am J Respir Crit Care Med 2015;191:1287-94. [Crossref] [PubMed]
- Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med 2013;187:99-105. [Crossref] [PubMed]
- Marshall NS, Wong KK, Cullen SR, et al. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med 2014;10:355-62. [PubMed]
- Chen JC, Hwang JH. Sleep apnea increased incidence of primary central nervous system cancers: a nationwide cohort study. Sleep Med 2014;15:749-54. [Crossref] [PubMed]
- Chang WP, Liu ME, Chang WC, et al. Sleep apnea and the subsequent risk of breast cancer in women: a nationwide population-based cohort study. Sleep Med 2014;15:1016-20. [Crossref] [PubMed]
- Christensen AS, Clark A, Salo P, et al. Symptoms of sleep disordered breathing and risk of cancer: a prospective cohort study. Sleep 2013;36:1429-35. [PubMed]
- Kendzerska T, Leung RS, Hawker G, et al. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ 2014;186:985-92. [Crossref] [PubMed]
- Nieto FJ, Peppard PE, Young T, et al. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2012;186:190-4. [Crossref] [PubMed]
- Martínez-García MA, Campos-Rodriguez F, Durán-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med 2014;15:742-8. [Crossref] [PubMed]
- Almendros I, Montserrat JM, Torres M, et al. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med 2012;13:1254-60. [Crossref] [PubMed]
- Almendros I, Montserrat JM, Torres M, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol 2013;186:303-7. [Crossref] [PubMed]
- Almendros I, Wang Y, Becker L, et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med 2014;189:593-601. [Crossref] [PubMed]
- Hakim F, Wang Y, Zhang SX, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res 2014;74:1329-37. [Crossref] [PubMed]
- Federico A, Morgillo F, Tuccillo C, et al. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer 2007;121:2381-6. [Crossref] [PubMed]
- Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2006;174:824-30. [Crossref] [PubMed]
- Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005;112:2660-7. [Crossref] [PubMed]
- Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch 2005;450:363-71. [Crossref] [PubMed]
- Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 2013;123:3664-71. [Crossref] [PubMed]
- Reinke C, Bevans-Fonti S, Drager LF, et al. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol (1985) 2011;111:881-90. [Crossref] [PubMed]
- Fitzpatrick SF, Tambuwala MM, Bruning U, et al. An intact canonical NF-kappaB pathway is required for inflammatory gene expression in response to hypoxia. J Immunol 2011;186:1091-6. [Crossref] [PubMed]
- Scholz CC, Cavadas MA, Tambuwala MM, et al. Regulation of IL-1beta-induced NF-kappaB by hydroxylases links key hypoxic and inflammatory signaling pathways. Proc Natl Acad Sci U S A 2013;110:18490-5. [Crossref] [PubMed]
- McElroy JA, Newcomb PA, Titus-Ernstoff L, et al. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res 2006;15:241-9. [Crossref] [PubMed]
- Gozal D, Farre R, Nieto FJ. Putative Links between sleep apnea and cancer: from hypotheses to evolving evidence. Chest 2015;148:1140-7. [Crossref] [PubMed]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014;41:49-61. [Crossref] [PubMed]
- Almendros I, Gileles-Hillel A, Khalyfa A, et al. Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: effect of tumor microenvironment. Cancer Lett 2015;361:233-9. [Crossref] [PubMed]
- Gharib SA, Seiger AN, Hayes AL, et al. Treatment of obstructive sleep apnea alters cancer-associated transcriptional signatures in circulating leukocytes. Sleep 2014;37:709-14, 714A-714T.
- Hogan AE, Corrigan MA, O'Reilly V, et al. Cigarette smoke alters the invariant natural killer T cell function and may inhibit anti-tumor responses. Clin Immunol 2011;140:229-35. [Crossref] [PubMed]
- Gaoatswe G, Kent BD, Corrigan MA, et al. Invariant Natural Killer T Cell Deficiency and Functional Impairment in Sleep Apnea: Links to Cancer Comorbidity. Sleep 2015;38:1629-34. [Crossref] [PubMed]


