Clinicopathological features and prognosis of primary pulmonary lymphoepithelioma-like carcinoma
Introduction
Lymphoepithelioma-like carcinoma (LELC) is one of the rare histological types of lung cancer, classified into one subtype of large cell carcinoma (LCC) (1), with the incidence of 1.15% to 5.90% of total LCC (2,3). It was reported that LELC was commonly found in the relatively young non-smoking Asian population, without a distinctive difference with respect to gender; more than half the patients were at an early stage (stage I and II) and were treated by complete resection (4).
The cause of LELC has, however, remained unclear. It was firstly reported by Bégin et al. that Epstein-Barr virus (EBV) was detected in the LELC tissue of a 40-year-old non-smoking Southeast Asian woman (5). The accumulated evidence indicated that Epstein-Barr virus-encoded RNA (EBER) was found in tumor cells (6,7) and EBV DNA was detectable in the serum of LELC patients (8), suggesting that the EBV infection was closely associated with the pathogenesis of LELC (9). The mutations of driver oncogenes were usually observed in lung cancers, whereas, rare or no mutations were found in the common oncogenes such as EGFR, KRAS, ALK, BRAF, ROS1, and p53 (7,10-13), implying that the mutagenesis of these genes was not involved in the tumorigenesis of LELC.
In the current study, a retrospective analysis on 43 LELC patients was conducted with respect to their clinical, pathological, and prognostic characteristics, in order to deeply investigate this rare subtype of lung cancer.
Methods
Patients
A total of 43 patients with LELC, confirmed by postoperative pathological examination, underwent the lung resection in Shanghai Chest Hospital between January 2010 and December 2015. The patients with the tumor metastasized from nasopharyngeal carcinoma or the organ tumors, and the patients with negative EBV by pathological test were excluded. The preoperative information of patients regarding the gender, age, diameter of tumor, smoking status, serum lung cancer biomarkers, and the neoadjuvant therapy history were collected. The treatment information such as the location of tumor observed intraoperatively, the invasion into the visceral pleura, and the mode of surgery were also collected. In addition, the shape of the resected tumor, cross-section characteristics, texture, boundary, lobular, necrosis, grading, stage, and the immunohistological results were recorded. The other data such as adjuvant therapy, disease severity, and survival were collected during the follow-up period. An informed consent was obtained from all patients before taking part in our study. The study was approved by the Institutional Review Board of Shanghai Chest Hospital [No. KS(Y)1531].
Methods
SPSS16.0 was utilized in the current study to perform the descriptive statistical analysis on the perioperative clinicopathological features of LELC. The survival and prognosis information collected during the follow-up period were analyzed by Kaplan-Meier method to calculate the overall survival (OS) and disease-free survival (DFS) rates. The significance of OS was assessed by the Log-rank test. The univariates and multivariates influencing the prognosis were analyzed by Cox regression. P<0.05 was considered as statistically significant.
Results
A total of 17,742 lung cancer patients underwent surgery and were diagnosed by postoperative pathological examination in Shanghai Chest Hospital between January 2010 and December 2015. Forty three patients were ultimately diagnosed as LELC, with the incidence of 0.25% in total lung cancers, including 20 men and 23 women. These patients were aged 57.35±9.22 years within the range of 30–78 years, including 6 smoking patients and 37 non-smokers. The diameters of tumors were 3.24±1.57 cm in average (range, 0.70–7.50 cm). There were 22 and 21 tumors located in the left and right lung, respectively. Twenty four tumors showed invasions into the visceral pleura; the shapes presented as blocks for 14 tumors and sphere for 29 tumors. The color of the tumor cross-sections exhibited as grey-white for 27. Thirty four tumors were hard textured. Forty tumors possessed unclear boundaries. There were 33 tumors without lobular; 40 tumors were not accompanied with necrosis, while the other 3 tumors had necrosis and 2 of them were accompanied with cavities, with diameters of 1 and 0.8 cm, respectively.
The surgery approach was determined according to the location of the tumor and intraoperative inspection, including wedge resection for 1 patient, segmental resection for 1 patient, lobectomy for 33 patients, lobectomy in combination with wedge resection for 1 patient, sleeve lobectomy for 2 patients, bilobectomy for 3 patients, and pneumonectomy for 3 patients. The limited wedge resection mentioned above was performed because a metastatic module in parietal pleura was found during operation. The UICC postoperative staging was determined according to the 7th edition published in 2009. Stage T included 8 of T1a, 6 of T1b, 21 of T2a, 5 of T2b, 2 of T3, and 1 of T4. Stage N included 23 of N0, 8 of N1, and 12 of N2. Stage M included 41 of M0 and 2 of M1a. The metastasis was not found preoperatively; however, the multiple metastatic modules in visceral pleura and a single metastatic module in parietal pleura were observed during the surgeries. Respectively, 10, 9, 8, 3, 11, and 2 patients were classified into stage Ia, Ib, IIa, IIb, IIIa, and IV according to TNM staging. Four patients received neoadjuvant therapy comprising of chemotherapy alone in three patients and radiotherapy only in one patient. Twenty eight patients underwent the preoperative serum tumor biomarker detection, including CYFRA21-1 positive for 12 patients, CA12-5 positive for 5 patients, NSE positive for 5 patients, SCC positive for 4 patients, and CEA positive for 2 patients; 12 patients were negative for all the above biomarkers. The immunohistological results for biomarkers were listed in Table 1.
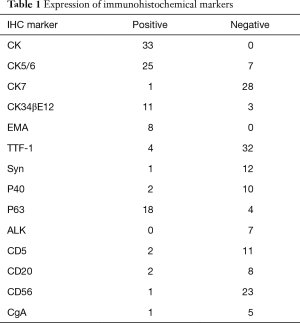
Full table
Forty patients were followed up after surgeries for 4 to 49 months (median time 30.5 months), and the other 3 patients lost the follow-up, with the total follow-up rate of 93.02%. One patient received postoperative radiotherapy alone, 14 patients received postoperative chemotherapy alone, and 10 patients received chemo-radiotherapy. Postoperative tumor metastasis site was listed in Table 2. Seven patients exhibited overall mortality, including tumor-related deaths in six patients and one from postoperative pulmonary infection in the opposite lung. Eight patients showed tumor-related survival despite of tumor metastasis. The other patients showed DFS. Kaplan-Meier analysis indicated that 2- and 5-year OS rates were 90% and 74%, respectively, whereas the DFS rates were 87% and 47%, respectively (Figure 1). The worse OS rate was indicated in the patients with higher grading and later stage tumors as compared to the patients with low grading and early stage tumors, according to the T and N grading and TNM staging by Log-rank test (P=0.025, P=0.002). The worse DFS was found in the patients with higher grading and later stage tumors (P=0.001, P=0.000) (Table 3). The T grading, N grading, and TNM phasing were the factors influencing the OS rate (P=0.047, P=0.017, P=0.008); the N grading and TNM phasing were also the factors that affect the OS rate (P=0.002, P=0.001) (Table 4) by Cox univariate analysis. However, no independent influencing factor of OS or DFS was found by multivariate analysis.

Full table

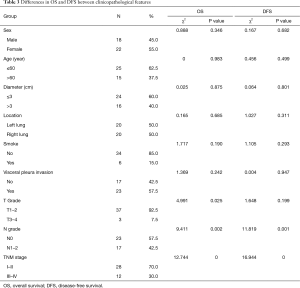
Full table

Full table
Discussion
LELC is a rare subtype of lung cancer. The patients were relatively young (13,14) and non-smokers (9,13-15). The incidence of LELC was not significantly different regarding gender (9). Our findings also indicated that the incidence was similar between males and females, with a slightly higher incidence in females (53.49%, 23/42). Nearly 86.05% (37/43) of patients were non-smokers, which was in agreement with the previously published study. However, the patients in the current study were relatively older with the average age of 57.35 years. LELC lacks specific manifestations as compared to the other subtypes of lung cancer; the symptoms such as cough, expectoration, chest tightness, and chest pain were found in the patients with large tumors, whereas, no overt symptoms could be manifested in that patient with small tumors that were coincidentally detected during the health checkup. The serum levels of cancer biomarkers were measured by Liang et al. (13) and 62.5% of CYFRA21-1 positive and 55% of NSE positive were found. In the current study, the positive rate for CYFRA21-1 was highest (42.86%, 12/28), while the positive rates for the other biomarkers were relatively lower.
The systematic preoperative examination was needed to exclude cancer that originated from the other organs, especially the nasopharynx. The imaging of LELC showed a lack of specificity, similar to the bronchial carcinoma (16). The average diameter of LELC reported by Ma et al. (7) was 4.1±1.9 cm; the average diameter measured by CT in the current study was 3.24±1.57 cm, slightly shorter than the published report. Sun et al. (14) demonstrated that LELC were commonly located in the left lung, which was similar to that in the current study wherein more tumors were found in the left lung (51.16%, 22/43). About 78.0% tumors were lobulated (17), whereas, the majority tumors (76.74%, 33/43) in the current study were non-lobular. It was still controversial whether the boundaries were distinct or not (16-18); our data indicated that 93.02% (40/43) LELC had unclear boundaries. The mass with thin wall and the smooth cavity were reported for some rare patients (19). In the current study, the cavity was found in 4.65% (2/43) tumors, with the diameter of 1.0 and 0.8 cm, respectively. The CT images were analyzed by Mo et al. (9) based on 35 LELC patients presenting solitary peripheral nodules in direct contact with adjacent pleura. Our findings indicated that 55.81% (24/43) LELC was not only in direct contact with pleura but also invaded into it.
The diagnosis of LELC was dependent on the pathological examination. A strong correlation between EBV infection and LELC has been reported in Asian patients as is the case of the patients included in the study, but may not be the case in Western populations (4,9,20). The presence of EBV (6,7,21) was tested in Asian patients as necessary because the EBV infection rate in Asian pulmonary LELC was as high as 94–100% (22,23). A large number of tumor cells were revealed by HE staining with the cellular proliferation rate of 80% accompanied by abundant plasma cells. The tumor cells had less cytoplasm with irregular nuclei and large nucleolus in a dark color. The lymphocytes infiltration into the tumor was observed with active mitosis. The tumor cells were presented as positive for CK, CK5/6, CK34βE12, Napsin A, and Bcl-2, but negative for CK7, CK14, CK20, EMA, TTF-1, CgA, Syn, and CD56 (6,7). Similar observations were made in the current study for positive rates of CK, CK5/6, and CK34βE12 staining, which were 100% (33/33), 78.13% (25/32), and 78.57% (11/14), respectively; the negative rates for CK7, TTF-1, CgA, Syn, and CD56 were 96.55% (28/29), 88.89% (32/36), 83.33% (5/6), 92.31% (12/13), and 95.83% (23/24), respectively. The positive rate of EMA was 100% (8/8), and that of P63 was 81.82% (18/22). The negative rates for P40, ALK, and CD20 were 83.33% (10/12), 100% (7/7), and 80% (8/10), respectively. The negative rate of CD5 in LCC was reported to be as high as 95.71% (21). Since LELC is one subtype of LCC, the negative rate of CD5 was also as high as 84.62% (11/13) in the current study.
The primary treatment method for LELC at an early stage was the radical resection; the chemotherapy, radiotherapy, and other comprehensive therapies were needed for the tumor at an advanced stage or with metastasis. The platinum/based chemotherapy can be used as the first-line of treatment for LELC at an advanced stage (24). The responsivity of LELC for 5-FU/folic acid/cisplatin treatment was 60% (25); capecitabine alone could be used as the salvage chemotherapy to maintain the stability of disease (26). Three patients received the platinum-based preoperative neoadjuvant chemotherapy, two with gemcitabine and cisplatin (GP) regime and one with pemetrexed and cisplatin (AP) regime. Twenty three patients received postoperative adjuvant chemotherapy, 17 with the platinum-based regime and 10 with GP regime. The chemotherapy strategy was adjusted according to the efficacy and reversed effects; 1 patient was switched to the biological immunotherapy because of intolerance for conventional chemotherapy and succumbed 1 year after surgery due to liver metastasis. The radiotherapy dosage should be determined according to the location of the tumor. The total dose for radiotherapy was suggested to be in the range of 5,000 and 7,000 cGy (24); the dose for each treatment was 200 cGy in the current study. One patient received preoperative neoadjuvant chemotherapy because of the potential risk of pleural invasion by the tumor located in the right upper lung. The other patients received local radiotherapy targeting the tumor recurrence or metastasis. Postoperative tumor metastasis occurred in 14 patients. After adjuvant chemo-radiotherapy, six patients died of cancer and eight patients survived. In the eight survivors, a long lasting metastatic mediastinal lymph node enlargement was found in one patient, and metastatic hilar and superior vena cava lymph nodes which considered as potential new metastasis sites were found after treatment of initial metastatic supraclavicular lymph node in another patient. The other six survived patients showed metastatic tumor partial response. The response rate to postoperative adjuvant therapy was 42.86% (6/14).
In general, the OS of LELC was better than that of other subtypes of non-small cell lung cancer (NSCLC) (4,24). The prognosis of the three LCC subtypes was compared by Sun et al., including 46 typical LCC, 30 large cell neuroendocrine carcinomas (LCNEC), and 18 LELC. LELC patients exhibited superior OS; the therapeutic outcome was also improved than the other two subtypes, and LELC was considered as an independent factor for prognosis (14). It was reported that the 2-year OS for different observations with 35, 52, and 74 LELC patients was 81%, 88%, and 86%, respectively, while the 5-year OS was 51%, 62%, and 72%, respectively (9,13,27). Moreover, the 5-year PFS and OS were 68% and 79%, respectively for the reports from Jiang et al. based on 79 LELC patients (28). Therefore, the prognosis of LELC was satisfactory. An improved OS could be found in the patients with the tumor at an early stage, without lymph node metastasis, and radical resection (13); the OS and DFS were worse for patients at the advanced stage of the tumor (27). It was reported that smoking (11) and staging of the tumor (11,28) were the two independent factors influencing OS as analyzed by COX regression; whereas, the diameter and the staging of the tumor (28) were the independent factors influencing DFS. In the current study, the prognosis was satisfactory, the 2- and 5-year OS were 90% and 74%, respectively and DFS were 87% and 47%, respectively. Further analysis indicated that OS was significantly poor in the patients with large tumor, lymph node metastasis, and at an advanced stage compared to those with a smaller tumor, without lymph node metastasis, and at an early stage. Univariate analysis also indicated that T grading, N grading, and TNM staging were the factors influencing OS. Significantly poor DFS was found in the patients with lymph node metastasis and at an advanced stage as compared to the patients without lymph node metastasis and at an early stage. Univariate analysis also indicated that N grading and TNM staging were the factors influencing DFS. However, no independent factor influencing OS or DFS was found by multivariate analysis.
Acknowledgements
Funding: This work was supported by Shanghai Rising Star Program (16QA1403500), Natural Science Foundation of Hubei Province (2014CFB420) and Independent Research Funding Project of Wuhan University (2042015kf0133 and 2042015kf0155).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Shanghai Chest Hospital [No. KS(Y)1531]. An informed consent was obtained from all patients before taking part in our study.
References
- Weissferdt A. Large cell carcinoma of lung: On the verge of extinction? Semin Diagn Pathol 2014;31:278-88. [Crossref] [PubMed]
- Liang R, Chen TX, Wang ZQ, et al. A retrospective analysis of the clinicopathological characteristics of large cell carcinoma of the lung. Exp Ther Med 2015;9:197-202. [PubMed]
- Pardo J, Martinez-Peñuela AM, Sola JJ, et al. Large cell carcinoma of the lung: an endangered species? Appl Immunohistochem Mol Morphol 2009;17:383-92. [Crossref] [PubMed]
- Ho JC, Wong MP, Lam WK. Lymphoepithelioma-like carcinoma of the lung. Respirology 2006;11:539-45. [Crossref] [PubMed]
- Bégin LR, Eskandari J, Joncas J, et al. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol 1987;36:280-3. [Crossref] [PubMed]
- Hayashi T, Haba R, Tanizawa J, et al. Cytopathologic features and differential diagnostic considerations of primary lymphoepithelioma-like carcinoma of the lung. Diagn Cytopathol 2012;40:820-5. [Crossref] [PubMed]
- Wang L, Lin Y, Cai Q, et al. Detection of rearrangement of anaplastic lymphoma kinase (ALK) and mutation of epidermal growth factor receptor (EGFR) in primary pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis 2015;7:1556-62. [PubMed]
- Ngan RK, Yip TT, Cheng WW, et al. Clinical role of circulating Epstein-Barr virus DNA as a tumor marker in lymphoepithelioma-like carcinoma of the lung. Ann N Y Acad Sci 2004;1022:263-70. [Crossref] [PubMed]
- Mo Y, Shen J, Zhang Y, et al. Primary lymphoepithelioma-like carcinoma of the lung: distinct computed tomography features and associated clinical outcomes. J Thorac Imaging 2014;29:246-51. [Crossref] [PubMed]
- Chang YL, Wu CT, Shih JY, et al. Unique p53 and epidermal growth factor receptor gene mutation status in 46 pulmonary lymphoepithelioma-like carcinomas. Cancer Sci 2011;102:282-7. [Crossref] [PubMed]
- Chang YL, Yang CY, Lin MW, et al. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: A potential rationale for immunotherapy. Lung Cancer 2015;88:254-9. [Crossref] [PubMed]
- Tam IY, Chung LP, Suen WS, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res 2006;12:1647-53. [Crossref] [PubMed]
- Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012;118:4748-58. [Crossref] [PubMed]
- Sun YH, Lin SW, Hsieh CC, et al. Treatment outcomes of patients with different subtypes of large cell carcinoma of the lung. Ann Thorac Surg 2014;98:1013-9. [Crossref] [PubMed]
- Huang CJ, Chan KY, Lee MY, et al. Computed tomography characteristics of primary pulmonary lymphoepithelioma-like carcinoma. Br J Radiol 2007;80:803-6. [Crossref] [PubMed]
- Hoxworth JM, Hanks DK, Araoz PA, et al. Lymphoepithelioma-like carcinoma of the lung: radiologic features of an uncommon primary pulmonary neoplasm. AJR Am J Roentgenol 2006;186:1294-9. [Crossref] [PubMed]
- Ma H, Wu Y, Lin Y, et al. Computed tomography characteristics of primary pulmonary lymphoepithelioma-like carcinoma in 4one patients. Eur J Radiol 2013;82:1343-6. [Crossref] [PubMed]
- Ooi GC, Ho JC, Khong PL, et al. Computed tomography characteristics of advanced primary pulmonary lymphoepithelioma-like carcinoma. Eur Radiol 2003;13:522-6. [PubMed]
- Hsieh MS, Wu CT, Chang YL. Unusual presentation of lymphoepithelioma-like carcinoma of lung as a thin-walled cavity. Ann Thorac Surg 2013;96:1857-9. [Crossref] [PubMed]
- Castro CY, Ostrowski ML, Barrios R, et al. Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopathologic study of 6 cases and review of the literature. Hum Pathol 2001;32:863-72. [Crossref] [PubMed]
- Kriegsmann M, Muley T, Harms A, et al. Differential diagnostic value of CD5 and CD117 expression in thoracic tumors: a large scale study of 1465 non-small cell lung cancer cases. Diagn Pathol 2015;10:210. [Crossref] [PubMed]
- Chen FF, Yan JJ, Lai WW, et al. Epstein-Barr virus-associated nonsmall cell lung carcinoma: undifferentiated "lymphoepithelioma-like" carcinoma as a distinct entity with better prognosis. Cancer 1998;82:2334-42. [Crossref] [PubMed]
- Han AJ, Xiong M, Gu YY, et al. Lymphoepithelioma-like carcinoma of the lung with a better prognosis. A clinicopathologic study of 32 cases. Am J Clin Pathol 2001;115:841-50. [Crossref] [PubMed]
- Huang CJ, Feng AC, Fang YF, et al. Multimodality treatment and long-term follow-up of the primary pulmonary lymphoepithelioma-like carcinoma. Clin Lung Cancer 2012;13:359-62. [Crossref] [PubMed]
- Ho JC, Lam WK, Wong MP, et al. Lymphoepithelioma-like carcinoma of the lung: experience with ten cases. Int J Tuberc Lung Dis 2004;8:890-5. [PubMed]
- Ho JC, Lam DC, Wong MK, et al. Capecitabine as salvage treatment for lymphoepithelioma-like carcinoma of lung. J Thorac Oncol 2009;4:1174-7. [Crossref] [PubMed]
- Wang L, Long W, Li PF, et al. An elevated peripheral blood monocyte-to-lymphocyte ratio predicts poor prognosis in patients with primary pulmonary lymphoepithelioma-like carcinoma. PLoS One 2015;10:e0126269. [Crossref] [PubMed]
- Jiang L, Wang L, Li PF, et al. Positive expression of programmed death ligand-1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma-like carcinoma. Onco Targets Ther 2015;8:1451-7. [PubMed]


