An unusual case of incomplete Carney triad: an 18-year-old girl suffering from multiple benign tumors
Case report
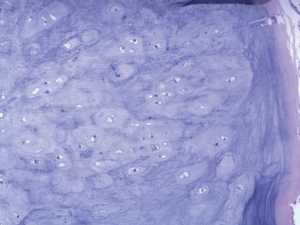
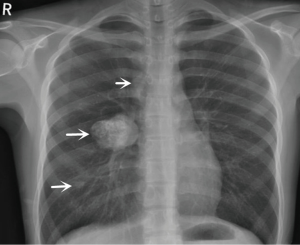
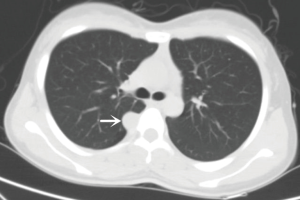
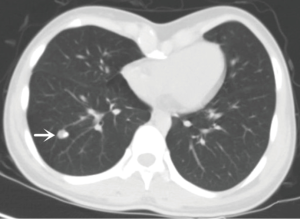
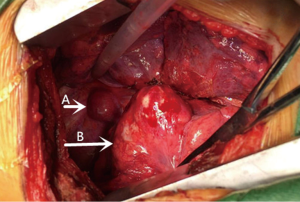
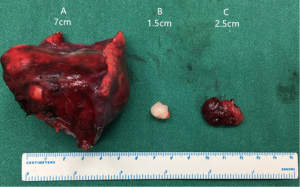
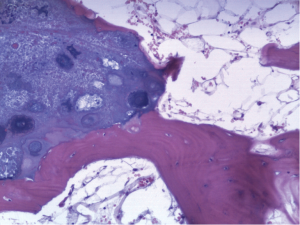
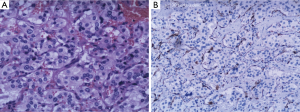
An 18-year-old girl was admitted into our hospital because of the continuous and irregular chest pain lasting for two months. In 2010, she received twice surgical resections of breast fibroma. In the same year, she was clinically diagnosed pulmonary hamartoma with diameter of about 4 cm in the hilum of her right lung. However, she did not receive any treatment. In 2011, she received the surgical resection of right lower limb chondroma, but her family history was uneventful. Chest X-ray after admission (Figure 1) showed two calcified shadows. One was about 5.2 cm × 4.5 cm near the right lung hilum and the other was about 0.9 cm × 0.7 cm in right lower lung. Besides, there was a hummocky shadow about 2.9 cm × 1.7 cm on the right side of the spine. Chest CT (Figure 2) showed that there was a soft and multiple calcified tissue mass about 5.8 cm × 3.1 cm near the bronchus of right lower lung, and its edge was clear and smooth. It is clinically diagnosed pulmonary hamartoma. As shown in Figure 3, there is a soft tissue mass with blood vessels likely connected to the descending aorta on the right side of fifth thoracic vertebra. Its unenhanced CT value was 42 HU and enhanced CT value was about 247 HU. Finally, it was diagnosed hypervascular neurogenic tumor located in posterior mediastinum. Figure 4 reveals a calcified nodule about 1.0 cm in basal segment of right lower lung, which was considered pulmonary chondroma or pulmonary hamartoma. Meanwhile, abdominal CT reveals no lesions. Paraganglioma and pulmonary hamartoma are respectively shown in Figure 5A,B. Operation was taken as follows: resection of dorsal segment of the right lower lung (Figure 6A), resection of nodule of basal segment of the right lower lung (Figure 6B) and resection of tumor of the posterior mediastinum (Figure 6C). Postoperative pathology: pulmonary hamartoma (dorsal segment of right lower lung, Figure 7), pulmonary chondroma (basal segments of right lower lung, Figure 8), paraganglioma leg (posterior mediastinum, Figure 9). Immunohistochemical results: CK (−), Vimentin (+), CgA (+), CD56 (+), Syn (+), s-100 sertoli cells (+), Actin (−), Desmin (−), Ki-67 (index of about 2%).

Discussion
Carney triad was firstly reported by Carney in 1977. It is a rare syndrome that involves gastrointestinal stromal tumor (GIST), pulmonary chondroma and extra-adrenal paraganglioma. Carney claimed that a person who just has two of these tumors can be diagnosed Carney triad, which is named “incomplete Carney triad” (1,2).
Carney triad is generally accepted to be a genetic disorder, but not a familial disease, because it is not passed on from generation to generation. Mosaicism and postzygotic mechanisms have been implicated. Some studies (3,4) have demonstrated the role of mitochondrial complex II abnormalities in Carney triad, as indicated by SDHB negative staining in tumors. These findings were further supported by recent studies (5-7) that provided evidences of the (rare) role of germline SDH genes mutation, and more frequently, SDHC hypermethylation in Carney triad.
The triad has preponderance in young females. In 2000, the research gathering 104 cases of Carney triad found more than 80% patients were women and most of them were under 30 years old. Besides, just 26% of the cases completely presented three types of tumors. The other cases only presented two tumors, mainly GIST and pulmonary chondroma, which accounted for 72.7% of all incomplete Carney triad cases (8).
Pulmonary chondroma of Carney triad is characterized as follows. Firstly, cartilage tissue of the tumor is myxoid or vitreous, and almost all calcified and (or) ossified. Secondly, the tumor is separated by fibrous capsule, and the boundaries of cartilage or bone tissue surrounded by fibrous pseudomembrane are clear to surrounding tissue. Moreover, the tumors are usually multiple but lack mitotic capacity and the ability of invasiveness or distant metastasis (9). Thus, the clinical symptom is mainly caused by tumors locally. GISTs of Carney triad are often multiple but only occur in the stomach, and most tumors locate in gastric antrum or body. The initial symptoms are non-specific, mainly including abdominal pain, gastrointestinal bleeding, anemia and weight loss (10,11). Carney triad is similar to sporadic GISTs both morphologically and immunologically. Actually, GIST in Carney triad is negative for KIT/PDGFRA mutation and SDHB expression (12-15), while most sporadic GISTs are positive for SDHB expression (97%) (3,4) and KIT/PDGFRA mutation (>80–85%). Most paragangliomas of Carney triad are functional and grow locally invasive. Therefore, there is a certain risk of distant metastases. It commonly occurs in aorticopulmonary body, carotid body, sympathetic trunk, and retroperitoneal organs, etc. Most patients presented clinical symptoms resulting of catecholamine releasing such as sweating, heart palpitations and so on. In particular, adolescent women with unknown anemia and hypertension should be wary of the possibility of Carney triad (16).
Nowadays, surgical resection is the major treatment for Carney triad. As the biological behavior of pulmonary chondroma is benign, it is feasible to enucleate tumor simply when the size of pulmonary chondroma is small. Similarly, pulmonary segmentectomy or lobectomy is necessary when the tumor is big enough. However, as there are multiple pulmonary chondromas, it is likely to recur in the event of simple excision of tumor (17). GIST of Carney triad shows malignant biological behavior that prones to distant metastasis like sporadic GIST. Therefore, it is necessary to sweep lymph node and resect omentum after total gastrectomy. For liver metastases, surgical resection is the preferred treatment. However, for unresectable metastases and recurrent GIST, it has been proved to be effective to take imatinib. The difference is hypertensive crisis which may occur while resecting paraganglioma (18). Patients whose tumors cannot be resected completely are advised to receive chemotherapy, radiotherapy or embolization polyvinyl alcohol particles (19).
The prognosis has been satisfied. In 1999, 79 cases were followed up from 1 to 49 years by Carney, and the result showed that the median survival time of 64 patients was 20 years. Likewise, Zhang followed up 104 cases of Carney triad patients in 2010, and the results showed that the patients’ survival rates of 10 and 40 years after operation were 100% and 73% respectively. However, because GIST and paraganglioma have malignant biological behavior, these patients need to be regularly monitored so as to guard against its recurrence and distant metastasis. It is reported that 18F-FDG is more helpful to discover early small abdominal metastases compared with MRI and 18F-DOPA (20). However, molecular imaging data about Carney triad is limited (21,22). During the 3-month follow-up, the girl has no recurrent lesions or evidence of new lesions. Long-term follow-up will be continuously taken in case of recurrence of tumors and the performance of other new tumors, especially GIST.
This article reported a case of incomplete Carney triad with pulmonary hamartoma. The patient is an 18-year-old girl who had the presentation of pulmonary chondroma and paraganglioma. In addition, the girl suffered breast fibroma and lower extremity chondroma. The patient merging multiple benign tumors is a coincidence or a special case of Carney triad. It is worth carrying on further research and analysis. Therefore, when a patient is founded a certain type of tumor presents systemically, attention should be paid to the possibility of Carney triad (23), especially for young women. A series of interviews with the sufferers are conductive to avoiding the missing Carney triad.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Carney JA, Sheps SG, Go VL, et al. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med 1977;296:1517-8. [Crossref] [PubMed]
- Slevin F, Duncan P, Shenjere P, et al. Two out of three required: a case of incomplete Carney triad. Int J Surg Pathol 2012;20:265-8. [Crossref] [PubMed]
- Gaal J, Stratakis CA, Carney JA, et al. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol 2011;24:147-51. [Crossref] [PubMed]
- Gill AJ, Chou A, Vilain R, et al. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am J Surg Pathol 2010;34:636-44. [PubMed]
- Haller F, Moskalev EA, Faucz FR, et al. Aberrant DNA hypermethylation of SDHC: a novel mechanism of tumor development in Carney triad. Endocr Relat Cancer 2014;21:567-77. [Crossref] [PubMed]
- Boikos SA, Xekouki P, Fumagalli E, et al. Carney triad can be (rarely) associated with germline succinate dehydrogenase defects. Eur J Hum Genet 2016;24:569-73. [Crossref] [PubMed]
- Killian JK, Miettinen M, Walker RL, et al. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci Transl Med 2014;6:268ra177. [Crossref] [PubMed]
- Carney JA. Gastric stromal sarcoma, pulmonary chondroma, and extra-adrenal paraganglioma (Carney Triad): natural history, adrenocortical component, and possible familial occurrence. Mayo Clin Proc 1999;74:543-52. [Crossref] [PubMed]
- Rodriguez FJ, Aubry MC, Tazelaar HD, et al. Pulmonary chondroma: a tumor associated with Carney triad and different from pulmonary hamartoma. Am J Surg Pathol 2007;31:1844-53. [Crossref] [PubMed]
- Zhang L, Smyrk TC, Young WF Jr, et al. Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol 2010;34:53-64. [Crossref] [PubMed]
- Carney JA. The triad of gastric epithelioid leiomyosarcoma, functioning extra-adrenal paraganglioma, and pulmonary chondroma. Cancer 1979;43:374-82. [Crossref] [PubMed]
- Diment J, Tamborini E, Casali P, et al. Carney triad: case report and molecular analysis of gastric tumor. Hum Pathol 2005;36:112-6. [Crossref] [PubMed]
- Spatz A, Bressac-de-Paillerets B, Raymond E. Soft tissue sarcomas. Case 3. Gastrointestinal stromal tumor and Carney's triad. J Clin Oncol 2004;22:2029-31. [Crossref] [PubMed]
- Knop S, Schupp M, Wardelmann E, et al. A new case of Carney triad: gastrointestinal stromal tumours and leiomyoma of the oesophagus do not show activating mutations of KIT and platelet-derived growth factor receptor alpha. J Clin Pathol 2006;59:1097-9. [Crossref] [PubMed]
- Agaimy A, Pelz AF, Corless CL, et al. Epithelioid gastric stromal tumours of the antrum in young females with the Carney triad: a report of three new cases with mutational analysis and comparative genomic hybridization. Oncol Rep 2007;18:9-15. [PubMed]
- Ssi-Yan-Kai G, Vial J, Labarre D, et al. A rare cause of anaemia associated with hypertension in a 14-year-old girl. Pediatr Radiol 2012;42:624-6. [Crossref] [PubMed]
- Wang W, Lai RQ, Chen XD, et al. Carney triad: a clinicopathological analysis of one case and review of the literature. Shijie Huaren Xiaohua Zazhi 2011;19:2794-7.
- Bladen JC, Moosajee M, Bassett JH. A tense case--Carney's triad. J R Soc Med 2004;97:540-1. [Crossref] [PubMed]
- Carney JA. Carney triad. Front Horm Res 2013;41:92-110. [Crossref] [PubMed]
- Papadakis GZ, Patronas NJ, Chen CC, et al. Combined PET/CT by 18F-FDOPA, 18F-FDA, 18F-FDG, and MRI correlation on a patient with Carney triad. Clin Nucl Med 2015;40:70-2. [Crossref] [PubMed]
- Kato K, Koike W, Iwano S, et al. Images of a case of Carney triad by combined F-18 FDG PET/CT. Clin Nucl Med 2011;36:698-700. [Crossref] [PubMed]
- Taïeb D, Sebag F, Sarde E, et al. First report of harlequin syndrome as the presenting feature of Carney Triad: a diagnostic and imaging challenge. J Clin Oncol 2012;30:e168-71. [Crossref] [PubMed]
- Chen CF, Chuang CH, Liu MK, et al. Clinical, radiologic and pathologic characteristics of the Carney triad: a case report and literature review. Kaohsiung J Med Sci 2010;26:428-34. [Crossref] [PubMed]