Image-guided thoracoscopic surgery with dye localization in a hybrid operating room
Introduction
As the National Lung Screening Trial demonstrating screening for lung cancer with chest computed tomography (CT) has reduced mortality from lung cancer (1,2), the use of low-dose CT screening is increasing and has increased the rate of detection of small lung nodules in asymptomatic patients. Most of these CT-detected lesions are indeterminate (3,4). A histopathological diagnosis is recommended when the nodule persists and has a solid component larger than 5 mm, or has enlarged on follow-up CT scans (5). However, an accurate diagnosis using CT-guided percutaneous biopsy is challenging, especially for lesions smaller than 2 cm with a predominately ground glass opacity (GGO) component (6,7). Transbronchial or transthoracic needle biopsy and positron emission tomography scans do not always allow definitive exclusion of malignancy in these lesions (8,9). Thus, patients with small indeterminate lung nodules and suspicious lung cancer lesions are often referred for surgical excision for both diagnostic and curative management.
Small lung lesions which do not cause surface abnormalities may not be identifiable under thoracoscopy. These lesions may require preoperative image-guided localization or wound extension for direct finger palpation, especially for GGOs or small lung nodules. In recent years, uniportal video-assisted thoracoscopic surgery (VATS) has grown in popularity because there is less postoperative pain and better patient satisfaction (10,11). However, finger palpation is not easy to perform under thoracoscopy through only one small incision, which makes preoperative localization even more essential for uniportal thoracoscopic surgery.
Our previous experience demonstrated the efficacy and safety of CT-guided dye localization at CT room followed by uniportal thoracoscopic surgery for resection of lung lesions (12,13). However, postprocedure complications such as pneumothorax, pulmonary hemorrhage, and hemoptysis increased the risks and the burden of nursing care for patients awaiting surgery, even though most of them received conservative management (14,15). By utilizing a hybrid operating room (OR), small lung lesions can be localized and resected precisely as a single procedure in the same place (16-19), which helps avoid the inherent risks of transfer after a transthoracic puncture, and reduces smudging of the injected dye.
Our aim is to provide a new workflow for image-guided dye localization using robotic C-arm CT before a thoracoscopic procedure in a hybrid OR, and evaluate its safety and feasibility.
Methods
Study design and patients
From July 2015 to July 2016, the medical records of patients who underwent image-guided dye localization of small pulmonary nodules (SPNs) using robotic C-arm CT immediately followed by thoracoscopic surgery in a hybrid OR were reviewed. The selection criteria for this procedure were patients with small peripheral lung nodules sized between 0.5 and 2.0 cm which would be difficult to identify during thoracoscopic surgery (pure or part-solid GGO lesions ≤2 cm, or solid nodules ≤1 cm). Patients requiring lobectomy or with deep lung nodules with depth >3 cm were excluded. This study was reviewed and approved by the National Taiwan University Hospital Research Ethics Committee (approval number: 201608041RIND).
Workflow
Hybrid OR setting
The hybrid OR is equipped with a robotic C-arm CT (Artis zeego, Siemens Healthcare GmbH, Forchheim, Germany). The robotic C-arm CT system is able to adapt to almost any position of the integrated free-floating surgical table such as tilted and cradled. The robotic C-arm CT system can be controlled near the surgical table or outside the hybrid OR.
Anesthesia and patient positioning
General anesthesia was performed on the free-floating table and the patients were intubated with either a double-lumen or single-lumen endotracheal tube. According to the location of the lung lesion, the patient was positioned in the supine or lateral decubitus position for the optimal access route for needle insertion and then fixed on the table with positioning aids (Figure 1A). During C-arm CT docking, all pipelines from the anesthesia side were gathered to the rostral axis and the C-arm was rotated laterally to check for any entanglement with the patient or lines.
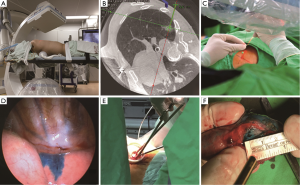
Localization with needle procedure
One of two radiologists (YC Chang, WC Ko) planned the needle routes and performed the dye-localization procedure. After making sure the C-arm could move freely around the patient, an initial C-arm CT volume was acquired (syngo DynaCT) for planning the intervention using a 6 sec scan protocol with 0.36 µGy/projection and 397 frames acquired over 200° during an end-inspiratory hold phase. This was initiated by clamping the endotracheal tube using a hemostat once the ventilator bellow provided the targeted tidal volume. After the protocol was completed, the hemostat was released and the patient resumed ventilation immediately. The access path was laid out in the isotropic data set using the syngo Needle Guidance of syngo X-Workplace (Siemens Healthcare GmbH, Forchheim, Germany). According to the initially defined paths, the skin entry point and target lesion were selected in syngo Needle Guidance, which then calculated the needle path (Figure 1B). During the needle procedure, the end-inspiratory hold phase was created and apnea was sustained 1 minute or longer according to the patient’s tolerance to secure the target lesion at the location shown on the last CT image. Before the needle procedure, the planned needle path was projected with a laser beam, making it possible to correct the position and direction of the needle. Under the end-inspiratory hold phase, a 22-gauge Chiba needle was gradually advanced to the planned depth under laser-supported guidance (Figure 1C). Thereafter, a second, control C-arm CT volume was acquired to evaluate the needle placement using an identical 6 sec scan protocol before cessation of the end-inspiratory hold phase. After confirmation of the optimal needle location, approximately 0.2 mL of PBV dye (patent blue V 2.5%; Guerbet, Aulnay-sous-Bois, France) was injected into the nodule. The last C-arm CT volume was acquired under the end-inspiratory hold phase to confirm immersion of the injected dye around the target. In some patients with deeply localized nodules, the subpleural lung parenchyma of the localization route was also localized with approximately 0.2 mL of PBV dye after additional C-arm CT confirmation.
Thoracoscopic surgery
After localization was completed, the anesthesiologist placed an endobronchial blocker or utilized a double lumen endotracheal tube to create one lung ventilation. The operation site was sterilized and draped for thoracoscopic surgery. Uniportal or multiportal thoracoscopic wedge resection was performed with the guidance of the blue dye (Figure 1D,E,F). If a frozen section of the lesion showed primary lung cancer, mediastinal lymphadenectomy was performed. An additional pulmonary resection was taken if the section margin was less than 2 cm. All incisions were closed, the chest was drained and the patient awakened, extubated, and transferred to the recovery room. The video of this section was shown in Figure 2.

Data collection and analysis
The demographic data, CT-localization results, anesthesia results, operative findings, and complications were retrospectively collected by chart review. Descriptive statistics are reported as median (range) for continuous data and as number (%) for categorical data.
Results
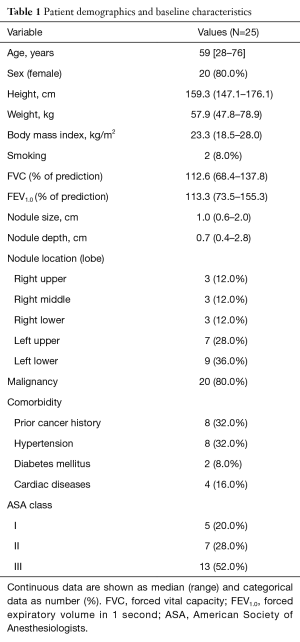
From July 2015 to July 2016, 25 consecutive patients with SPNs smaller than 2 cm underwent robotic C-arm CT-guided blue dye tattooing followed by immediate VATS in a hybrid OR. The demographics and baseline characteristics are reported in Table 1. Their median age was 59 years and 20 (80%) were women. Their median body mass index was 23.3 kg/m2. The median tumor size was 10 mm. The pulmonary function of the patients was generally good, with a median forced expiratory volume in 1 second of 113% of predicted. Coexisting medical diseases were present in 17 patients. At the time of the procedure, the American Society of Anesthesiologists Physical Status Classification was class 1 in 5 patients, class 2 in 7, and class 3 in 13.
Full table
Twenty patients (80%) received single lumen endotracheal tube intubation with an endobronchial blocker and the others received a double lumen for general anesthesia and lung separation. Twenty-one patients (84%) were positioned in the lateral decubitus (or semi-prone) position and 4 were supine for the localization procedure. Twenty-three patients (92%) had successful intraoperative dye injection to localize the SPN. Figure 3 demonstrates images of C-arm CT-guided dye localization. The blue dye immersed around the lesions in these patients was visible on the pleural surface of the lung at thoracoscopy (Figure 1D). Two patients (8%) had unexpected events during the needle localization procedure, and both of them received thoracoscopic surgery without adequate localization. One patient with a lesion over the right middle lobe had a puncture through the diaphragm into the liver parenchyma. The other patient with two lesions over the left lower lobe had a large pneumothorax after dye injection for the first lesion. The CT-guided localization procedures were discontinued when these events were noted and thoracoscopic surgery was performed. The lung nodules were identified with the conventional palpation method after extension of the wounds. One patient required addition pulmonary resection because the safety margin of wedge resection was less than 2 cm.
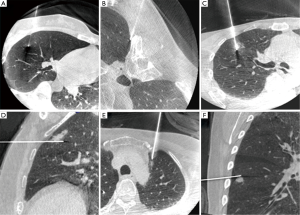
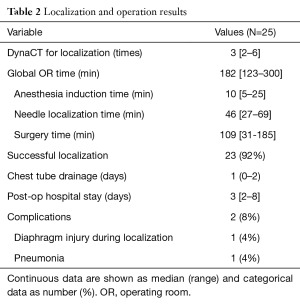
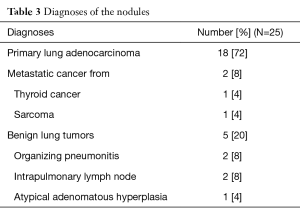
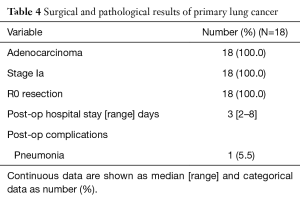
Table 2 showed the results of localization and operation. The median length of the global operation time, defined as the whole duration of the patient in the OR, was 182 (range, 123–300) min. The median duration of anesthesia induction before localization was 10 (range, 5–25) min. The median localization time measured from patient positioning with C-arm docking to the end of the needle procedure was 46 (range, 27–69) min. The median duration of thoracoscopic surgery was 109 (range, 31–185) min. Syngo DynaCT was performed intraoperatively an average of 3.6 times (median: 3 times), and the median durations of postoperative chest tube drainage and hospital stay were 1 and 3 days, respectively. Table 3 provides the pathological diagnoses of the 25 nodules resected. Eighteen (72%) were primary lung adenocarcinomas, all of which were stage Ia. Two (8%) metastatic lesions were reported and benign disease was diagnosed in 5 (20%) lesions with two organizing pneumonitis, two intrapulmonary lymph nodes, and one atypical adenomatous hyperplasia. Table 4 provides the surgical and pathological outcomes of the primary lung cancers in this cohort.
Full table
Full table
Full table
Discussion
In recent years, robotic C-arm CT-guided interventions have been used more frequently because of the improving image quality of flat-panel CT and the availability of three-dimensional mapping and navigation applications. A robotic C-arm CT interventional suite can provide real-time visualization for transthoracic needle biopsy (TNB) and more flexibility of the detector system around the patient compared with conventional gantry-based, multidetector CT (MDCT) (21-24). Furthermore, the patient has not to be repositioned and the system complies even with orthopedic hygienic standards. Rotolo et al. (21) evaluated 324 TNBs using either C-arm CT or MDCT and demonstrated that C-arm CT-guided TNB provided high and comparable diagnostic accuracy (96% vs. 94%) with a similar radiation dose. However, MDCT guidance is only possible in an axial plane, thus making off-plane interventions more difficult and time consuming, whereas, the C-arm CT system is not restricted to a single plane (22). Thus, the C-arm CT guidance enables better angle flexibility for nodule localization, especially in decubitus position.
Several preoperative localization methods, including dye injection (14,25,26), hookwire placement (27-30), and coil labeling (31), have been described for SPNs, and these methods have their advantages and disadvantages. Because hookwire dislodgment and coil migration may occur during patient transfer, we prefer to use dye injection routinely. We initially used methylene blue dye for preoperative CT-guided localization, however, the dye rapidly diffused into the surrounding lung parenchyma in some patients. Therefore, we began using PBV dye for pulmonary nodule localization in 2013, and the localization success rate in our institution was 100% in patients with a single nodule and 97.2% in those with synchronous multiple nodules (25). In our experience, preoperative CT-guided PBV dye localization also contributes to a satisfactory operative outcome for SPNs managed with uniportal VATS (12,13), which comprised 80% of cases in our cohort. In this study, the success rates of localization using PBV injection under C-arm CT guidance was 92% which is slightly lower than MDCT guidance (25). We speculate that the learning curve using C-arm CT guidance and limited case number may contribute the difference. Also the C-arm CT patients were intubated with positive ventilation, which may lead to a higher risk of a large pneumothorax during a needle procedure.
Several groups have demonstrated successful combination of intraoperative CT-guided localization and immediate thoracoscopic surgery for SPNs (16-18). Narayanam et al. (16) described a series of 31 pediatric patients with a total of 34 SPNs receiving lung tattooing with immediate video-assisted thoracoscopic resection performed as a single procedure in a hybrid room. Lung tattooing was performed under conventional gantry-based MDCT. The technical success rate was 91.1% and the diagnostic yield was 100%. Ohtaka et al. (17) used mobile CT for intraoperative pulmonary nodule localization. Ten patients with small lung nodules underwent VATS using an intraoperative imaging device with a flat-panel detector that provides CT imaging. The positional relationship between the needle marking and the tumor was recognized intraoperatively. In 9 patients (90%), the tumor could be seen on intraoperative CT images using their mobile CT.
Another group from Harvard Medical School (18) developed a prospective phase I-II clinical trial to facilitate localization and resection of small lung nodules. In their study, an intraoperative C-arm CT scan was utilized for guidance of percutaneous marking with T-bars followed by VATS resection of the tumor. All 23 patients underwent complete resection of their lesions and CT imaging of the resected specimens confirmed the removal of the T-bars and the nodule. The median time required for placement of the two T-bars and for the VATS were 39 and 67 min, respectively, which saved a lot of time compared with the first MDCT-based series and also our results (average localization procedure: 33 min; VATS: 109 min).
Selection of a hybrid OR table depends on the primary use of the system. Surgeons, particularly orthopedic, general, and neurosurgeons, usually expect a segmented tabletop for flexible patient positioning (32,33). Thoracic surgeons also require a flexible table to help bridging, which is opening up the intercostal spaces (ICS) when the patient is in the lateral decubitus position. However, most hybrid ORs were originally set up for cardiac and vascular procedures and are usually equipped with floatable angiography tables. Ng et al. (19) described their substitution for bridging by placing a rolled up pillow under the patient’s mid thorax to open up the ICS when doing an image-guided localization uniportal lobectomy in a hybrid OR. In our series, the patients were positioned laterally without trying to open up the ICS. Patients with anticipated anatomical pulmonary resections were excluded from our project, because those procedures are thought to be more difficult and require standard surgical tables. Measures must be taken to avoid touching the patient and entangling accessory lines and tubing during C-arm rotation before acquiring syngo DynaCT images. Ng et al. (19) suggested that the anesthesia equipment be placed further from the patient than usual to ensure collision-free C-arm rotation. This may require extension tubing. In our practice, the anesthesia equipment is located as usual, and all tubes and lines are secured together extending from the patient to the machine through the axis of the table, preventing entanglement with the rotating C-arm.
In our series, one patient had a failed localization due to large pneumothorax noted on real-time imaging after the needle puncture. Under positive pressure ventilation, the incidence of a large pneumothorax after thoracic needle procedures may rise compared with procedures done under spontaneous breathing. In future practice, we will use catheter drainage or create a thoracic incision as salvage for a large pneumothorax during needle localization to complete the procedure. Another patient had a diaphragm puncture into the liver parenchyma, which is usually encountered with lesions located at the basal portion close to the diaphragm. With lesions at the basal portion, care should be taken before the needle enters the pleural cavity. Syngo DynaCT should be performed to prevent unexpected diaphragm puncture when the needle tip is still in the chest wall. Alternative localization methods without puncture into the pleural cavity have been described in Japanese centers (34,35). These can also be considered to avoid complications.
The success of this program depends on close cooperation between different specialties, including anesthesiologists, intervention radiologists, technicians, and surgeons. To gather these experts at a scheduled time to go through a time-consuming process in the hybrid OR, which was in paucity and usually controlled by cardiovascular specialist, is the most challenging part. For the practitioners wishing to start such a program in their own institution, we suggest starting for peripheral and probably palpable lesions in case of unsuccessful localization, and to avoid lesions near the diaphragm, at the base of lower lobe, or area covered by scapula. A special consideration should be focused on the general anesthesia during needle localization. Although some of the center prefer to maintain contralateral lung ventilation during needle localization, our workflow suggest totally apnea with inflated lung for better image acquisition and accuracy of localization.
Conclusions
In conclusion, we presented a new workflow for image-guided dye localization with an immediate thoracoscopic procedure for SPNs in a hybrid OR. Through this multidisciplinary approach, patients can be safely managed with successful resection for both diagnostic and therapeutic needs in one stop under a single anesthetic.
Acknowledgements
The authors would like to thank Siemens Healthcare Research Collaboration Scientist Frank Chun-Hsien Wu, PhD, and Application Specialist Maxwell Jiun-Yan Lin for their technical assistance with Artis zeego operation and image post-processing.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed and approved by the National Taiwan University Hospital Research Ethics Committee (approval number: 201608041RIND).
References
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Krochmal R, Arias S, Yarmus L, et al. Diagnosis and management of pulmonary nodules. Expert Rev Respir Med 2014;8:677-91. [Crossref] [PubMed]
- Raad RA, Suh J, Harari S, et al. Nodule characterization: subsolid nodules. Radiol Clin North Am 2014;52:47-67. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [Crossref] [PubMed]
- Yeow KM, Tsay PK, Cheung YC, et al. Factors affecting diagnostic accuracy of CT-guided coaxial cutting needle lung biopsy: retrospective analysis of 631 procedures. J Vasc Interv Radiol 2003;14:581-8. [Crossref] [PubMed]
- Kothary N, Lock L, Sze DY, et al. Computed tomography-guided percutaneous needle biopsy of pulmonary nodules: impact of nodule size on diagnostic accuracy. Clin Lung Cancer 2009;10:360-3. [Crossref] [PubMed]
- Kozower BD, Meyers BF, Reed CE, et al. Does positron emission tomography prevent nontherapeutic pulmonary resections for clinical stage IA lung cancer? Ann Thorac Surg 2008;85:1166-9; discussion 1169-70. [Crossref] [PubMed]
- Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. [Crossref] [PubMed]
- Chen PR, Chen CK, Lin YS, et al. Single-incision thoracoscopic surgery for primary spontaneous pneumothorax. J Cardiothorac Surg 2011;6:58. [Crossref] [PubMed]
- Hung MH, Cheng YJ, Chan KC, et al. Nonintubated uniportal thoracoscopic surgery for peripheral lung nodules. Ann Thorac Surg 2014;98:1998-2003. [Crossref] [PubMed]
- Hung WT, Hsu HH, Hung MH, et al. Nonintubated uniportal thoracoscopic surgery for resection of lung lesions. J Thorac Dis 2016;8:S242-50. [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- Gossot D, Miaux Y, Guermazi A, et al. The hook-wire technique for localization of pulmonary nodules during thoracoscopic resection. Chest 1994;105:1467-9. [Crossref] [PubMed]
- Narayanam S, Gerstle T, Amaral J, et al. Lung tattooing combined with immediate video-assisted thoracoscopic resection (IVATR) as a single procedure in a hybrid room: our institutional experience in a pediatric population. Pediatr Radiol 2013;43:1144-51. [Crossref] [PubMed]
- Ohtaka K, Takahashi Y, Kaga K, et al. Video-assisted thoracoscopic surgery using mobile computed tomography: new method for locating of small lung nodules. J Cardiothorac Surg 2014;9:110. [Crossref] [PubMed]
- Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial. J Surg Oncol 2015;112:18-25. [Crossref] [PubMed]
- Ng CS, Man Chu C, Kwok MW, et al. Hybrid DynaCT scan-guided localization single-port lobectomy. Chest 2015;147:e76-8. [Crossref] [PubMed]
- Yang SM, Ko WC, Lin MW, et al. This video presented the workflow of image-guide VATS in hybrid OR, which include the following steps. Asvide 2016;3:397. Available online: http://www.asvide.com/articles/1168
- Rotolo N, Floridi C, Imperatori A, et al. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur Radiol 2016;26:381-9. [Crossref] [PubMed]
- Kostrzewa M, Rathmann N, Kara K, et al. Accuracy of percutaneous soft-tissue interventions using a multi-axis, C-arm CT system and 3D laser guidance. Eur J Radiol 2015;84:1970-5. [Crossref] [PubMed]
- Gupta R, Cheung AC, Bartling SH, et al. Flat-panel volume CT: fundamental principles, technology, and applications. Radiographics 2008;28:2009-22. [Crossref] [PubMed]
- Jiao D, Yuan H, Zhang Q, et al. Flat detector C-arm CT-guided transthoracic needle biopsy of small (≤2.0 cm) pulmonary nodules: diagnostic accuracy and complication in 100 patients. Radiol Med 2016;121:268-78. [Crossref] [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-544.e2. [Crossref] [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Chen YR, Yeow KM, Lee JY, et al. CT-guided hook wire localization of subpleural lung lesions for video-assisted thoracoscopic surgery (VATS). J Formos Med Assoc 2007;106:911-8. [Crossref] [PubMed]
- Seo JM, Lee HY, Kim HK, et al. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg 2012;143:809-14. [Crossref] [PubMed]
- Lizza N, Eucher P, Haxhe JP, et al. Thoracoscopic resection of pulmonary nodules after computed tomographic-guided coil labeling. Ann Thorac Surg 2001;71:986-8. [Crossref] [PubMed]
- Bonatti J, Vassiliades T, Nifong W, et al. How to build a cath-lab operating room. Heart Surg Forum 2007;10:E344-8. [Crossref] [PubMed]
- Nollert G, Wich S. Planning a cardiovascular hybrid operating room: the technical point of view. Heart Surg Forum 2009;12:E125-30. [Crossref] [PubMed]
- Nishida T, Fujii Y, Akizuki K. Preoperative marking for peripheral pulmonary nodules in thoracoscopic surgery: a new method without piercing the pulmonary parenchyma. Eur J Cardiothorac Surg 2013;44:1131-3. [Crossref] [PubMed]
- Kawada M, Okubo T, Poudel S, et al. A new marking technique for peripheral lung nodules avoiding pleural puncture: the intrathoracic stamping method. Interact Cardiovasc Thorac Surg 2013;16:381-3. [Crossref] [PubMed]


