Birt-Hogg-Dube syndrome accompanied by pulmonary arteriovenous malformation
A 25-year-old male visited a nearby clinic due to sudden chest pain. A chest X-ray revealed the presence of fluid in his left lung and was subsequently sent to our hospital. He did not have any significant medical history and has been living a healthy life. However, his family history indicated that his mother, mother’s cousin, and maternal grandfather each suffered from pneumothorax, and his maternal grandfather suffered from colon cancer. Physical findings confirmed the presence of numerous small papules on his nose and neck regions; however, no other symptoms were found.
Blood work showed increased inflammation values (WBC: 12,700/uL and CRP: 2.37 mg/dL), although a tumor marker showed only a slightly increased value (CEA: 5.0 ng/mL). No anemia was detected.
A chest X-ray revealed fluid in his left lung and a tumor shadow that overlapped with his heart shadow were also observed. A chest CT identified focal emphysematous changes in both lungs and a tumor with enhanced contrast, which was approximately 5 cm in diameter, in the posterior basal segment of left lung with pleural fluid (Figure 1A,B).
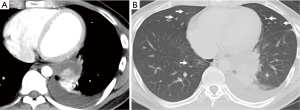
Bloody pleural fluid was identified by pleural fluid tap; therefore, we considered the possibility of hemothorax caused by a hemorrhage from a tumor-like lesion. Surgery was recommended for hemostasis to treat the hemothorax.
Surgery
Thoracoscopic findings were a large amount of dark red pleural fluid and multiple nonspecific cysts in the lung. The presence of a black tumor-like lesion that was adhered to the diaphragm was found in the posterior basal segment of left lung and was resected. Rapid operative diagnosis indicated that the black tumor-like lesion was an intrapulmonary hematoma, no malignancy.
Pathology
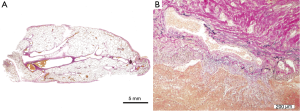
The tumor-like lesion was caused by a significant hemorrhage that occurred in the pulmonary parenchyma, which was diagnosed as intrapulmonary hematoma. Aggregated vascular vessels of various diameters were found in the area surrounding the intrapulmonary hemorrhage. These vascular vessels contained an immature fibrous wall of tunica media was subsequently diagnosed as pulmonary arteriovenous malformation (Figure 2A,B). Pulmonary cysts characteristic of Birt-Hogg-Dube (BHD) syndrome were not found in the resected sample.
Genetic test
Family history of the patient indicated the presence of pneumothorax and malignant diseases; moreover, small papules were observed on patient’s skin. Chest CT showed multiple pulmonary cysts, indicating the possibility of BHD syndrome. To confirm, we obtained informed consent from the patient to perform a genetic test. The genetic test revealed a deletion of exon 11 of the BHD gene; thus, he was diagnosed with BHD syndrome.
Discussion
BHD syndrome is an autosomal dominant inherited disorder by a mutation in the FLCN gene on 17p11.2. (1). It has three key characteristics, which are cutaneous fibrofolliculomas, renal cell tumors, and lung cysts (1,2). It is known that the lung diseases associated with BHD syndrome are multiple pulmonary cysts and spontaneous pneumothorax. According to chest CT, pulmonary cysts and pneumothorax are found in 90% and 24% of patients with BHD syndrome, respectively. Pathological characteristics of lung diseases include basilar cysts comprised of intraparenchymal collections of air that are surrounded by either normal parenchyma or a thin fibrous wall and blebs consisting of collections of air within the pleura (3). Although these pathological findings are not specific to BHD syndrome, their predominantly basilar location contrasts with the apical distribution of other more well-recognized causes of spontaneous pneumothorax, such as emphysematous bullae and idiopathic blebs. The causes of pulmonary cysts are not well understood.
Pulmonary arteriovenous malformation is associated with congenital abnormal fistula of the pulmonary blood vessels (4,5). It is a relatively rare disease and is often diagnosed when hemoptysis is detected. The rate of intrapulmonary hemorrhage caused by pulmonary arteriovenous malformation is between 1% to 7% (4,5); therefore, there is the possibility of a massive hemorrhage, which is a fatal complication that requires treatment in the early stages in order to prevent. Catheter embolization and surgical resection are methods for treating pulmonary arteriovenous malformation (5). In this study, it was difficult to diagnose pulmonary arteriovenous malformation before the surgery; therefore, pulmonary arteriovenous malformation was treated by resecting the affected area.
The present case study is one example of BHD syndrome, which was discovered due to an intrapulmonary hemorrhage caused by pulmonary arteriovenous malformation. Association of pulmonary arteriovenous malformation with BHD syndrome has not been previously reported; therefore, the relationship is unknown. Kapoor et al. hypothesize that mutated FLCN may affect hypoxia-inducible factor 1-alpha (HIF-1α) activation and implicate in vascular malformation on intracranial vascular pathology (6). Clarification of the relationship between pulmonary arteriovenous malformation and BHD syndrome is anticipated through the accumulation of more BHD syndrome cases.
Acknowledgements
The authors thank to Kuniaki Seyama MD, PhD. Department of Respiratory Medicine, Juntendo University School of Medicine, for the genetic test of the BHD gene.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell 2002;2:157-64. [Crossref] [PubMed]
- Adley BP, Smith ND, Nayar R, et al. Birt-Hogg-Dubé syndrome: clinicopathologic findings and genetic alterations. Arch Pathol Lab Med 2006;130:1865-70. [PubMed]
- Butnor KJ, Guinee DG Jr. Pleuropulmonary pathology of Birt-Hogg-Dubé syndrome. Am J Surg Pathol 2006;30:395-9. [Crossref] [PubMed]
- Cottin V, Dupuis-Girod S, Lesca G, et al. Pulmonary vascular manifestations of hereditary hemorrhagic telangiectasia (rendu-osler disease). Respiration 2007;74:361-78. [Crossref] [PubMed]
- Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med 1998;158:643-61. [Crossref] [PubMed]
- Kapoor R, Evins AI, Steitieh D, et al. Birt-Hogg-Dubé syndrome and intracranial vascular pathologies. Fam Cancer 2015;14:595-7. [Crossref] [PubMed]


